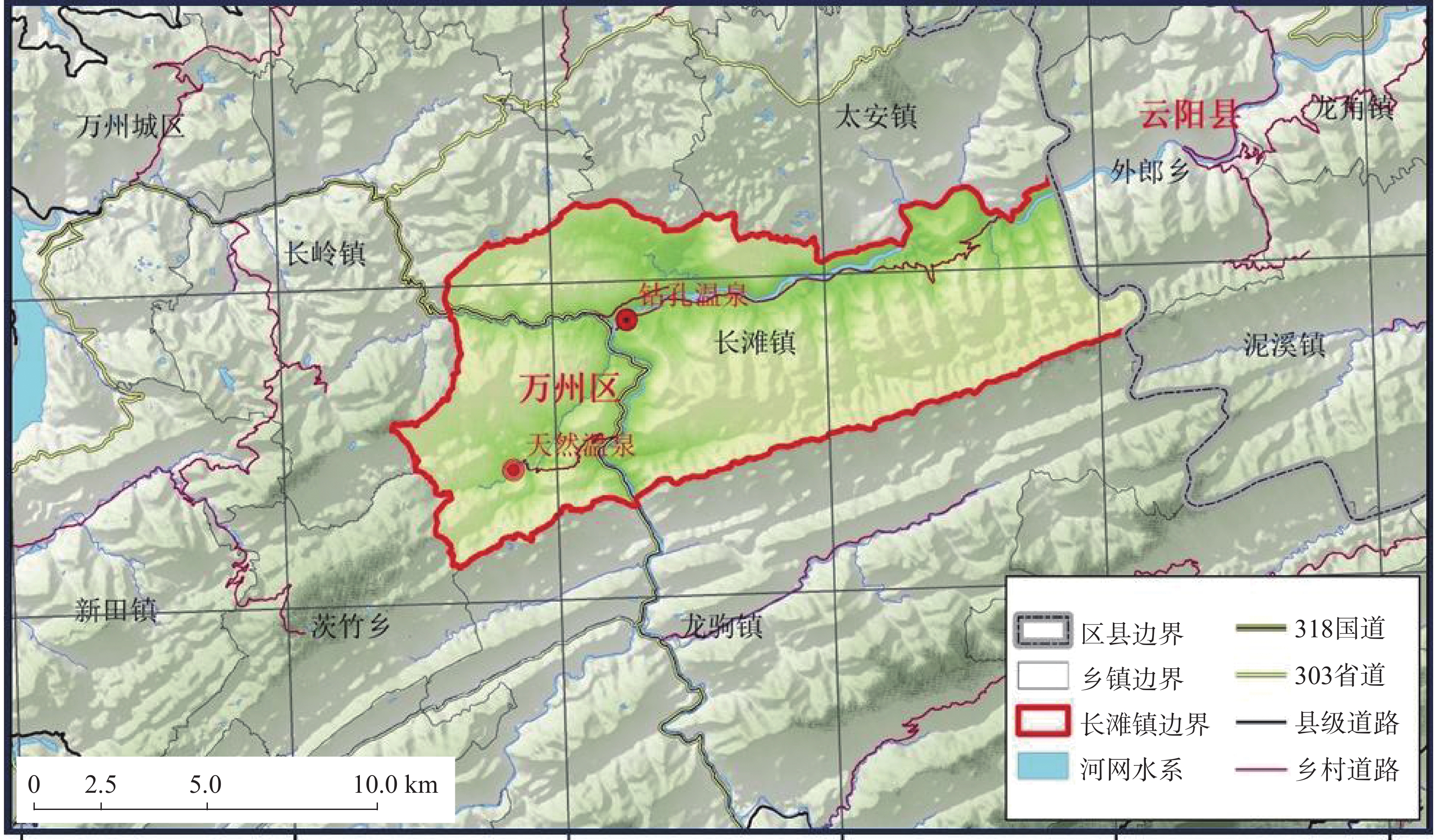
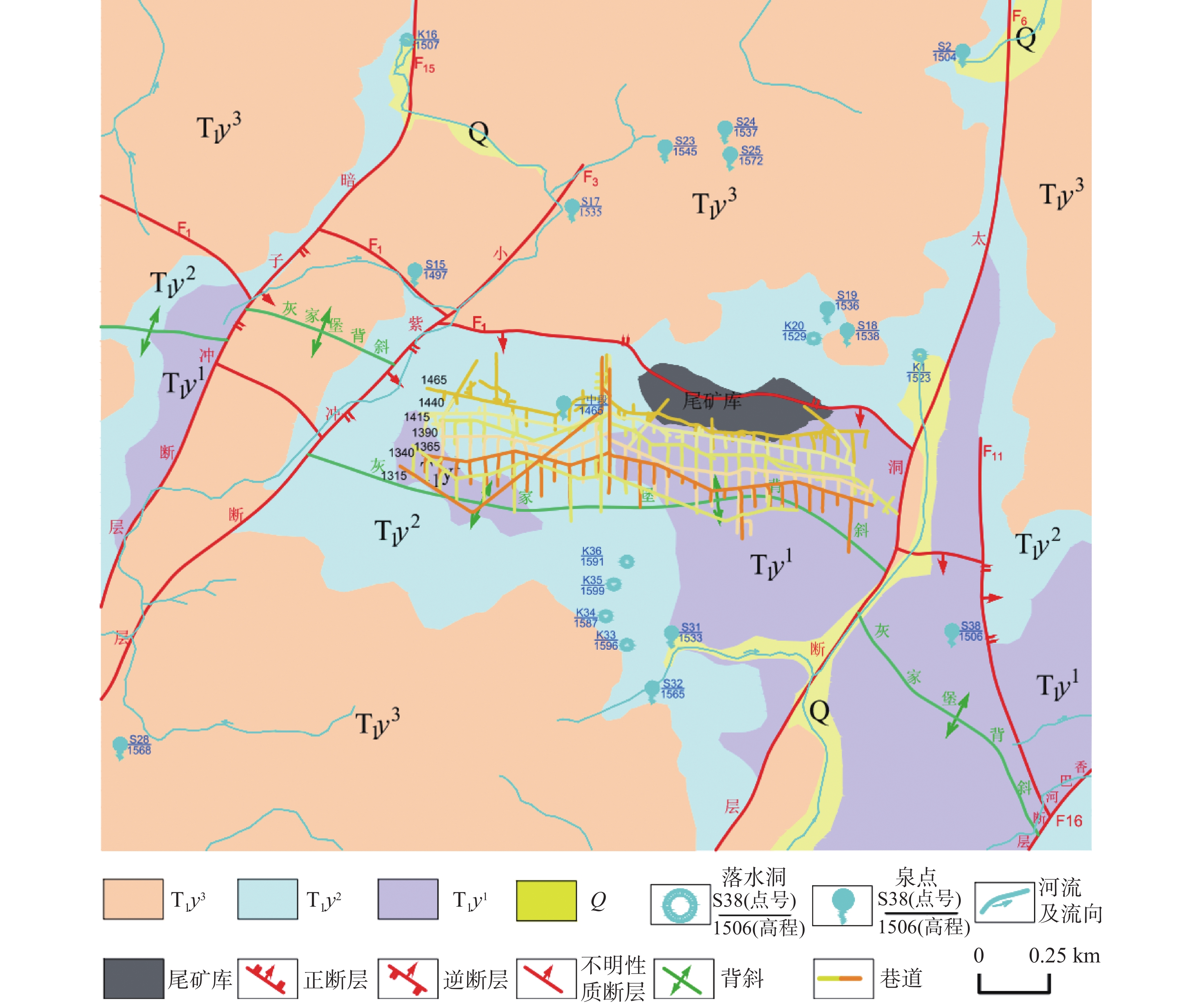
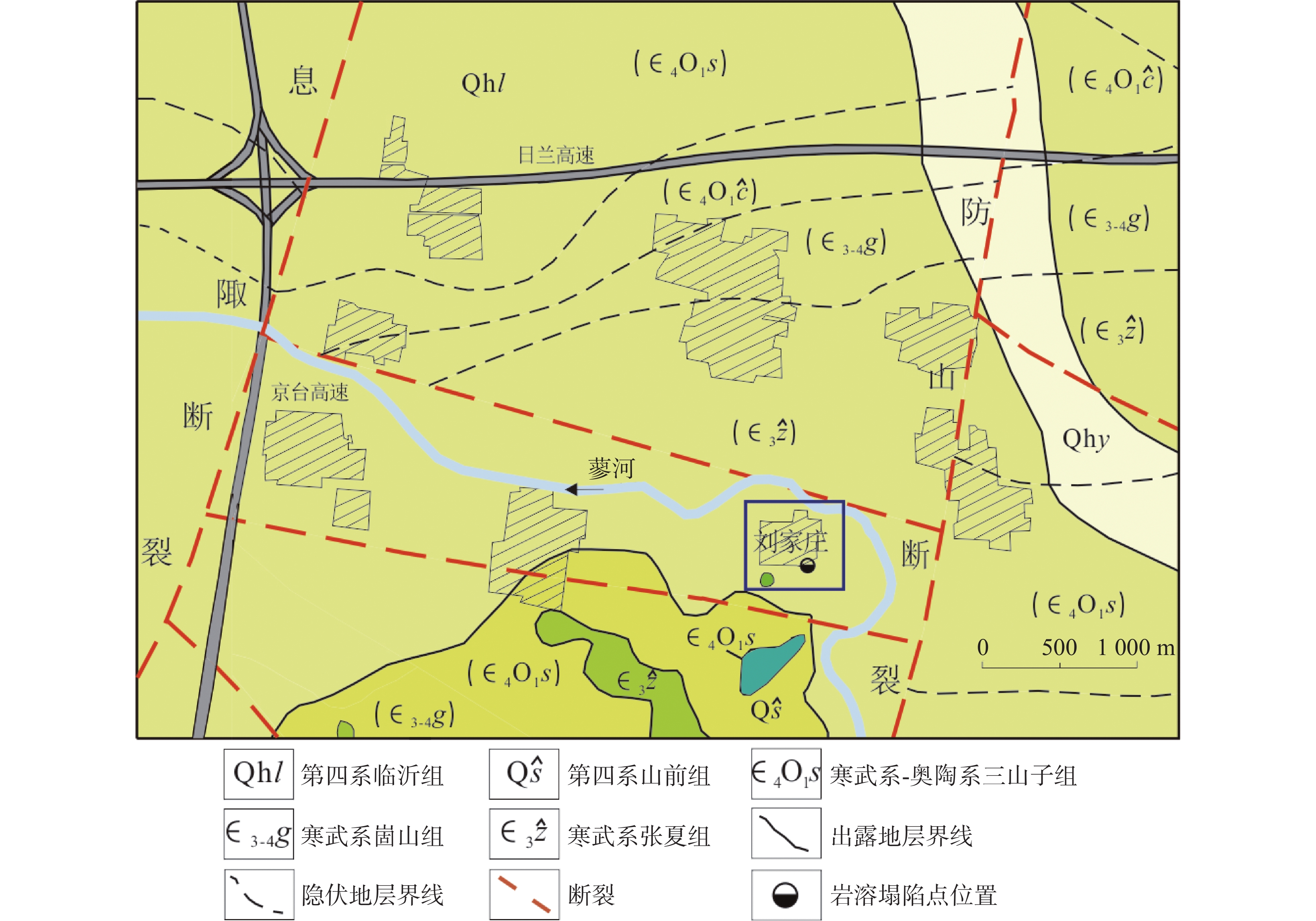
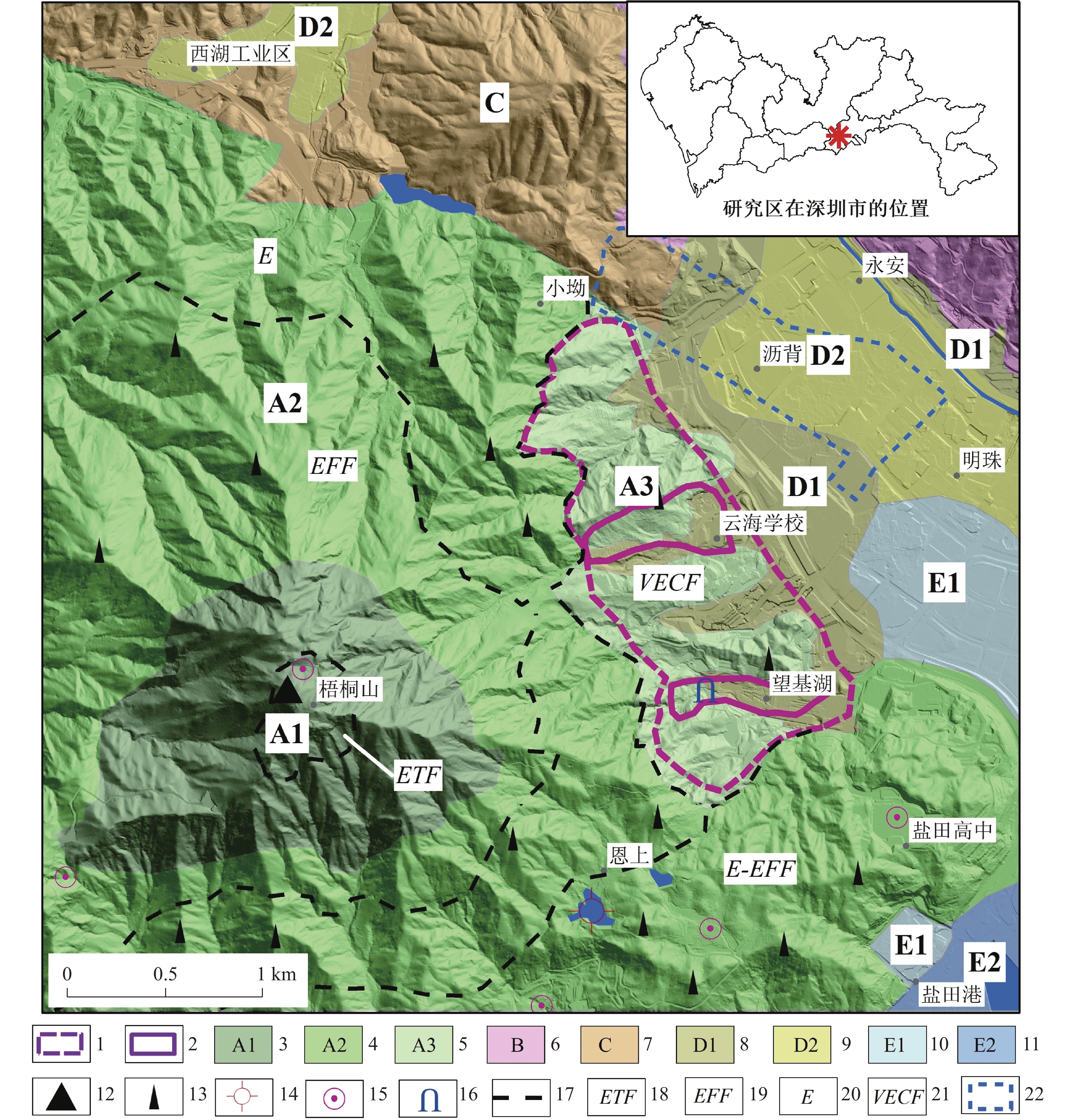
Evaluation results, issues, and prospects of investigations into groundwater, mineral water, and geothermal (water) resources in Yunnan Province
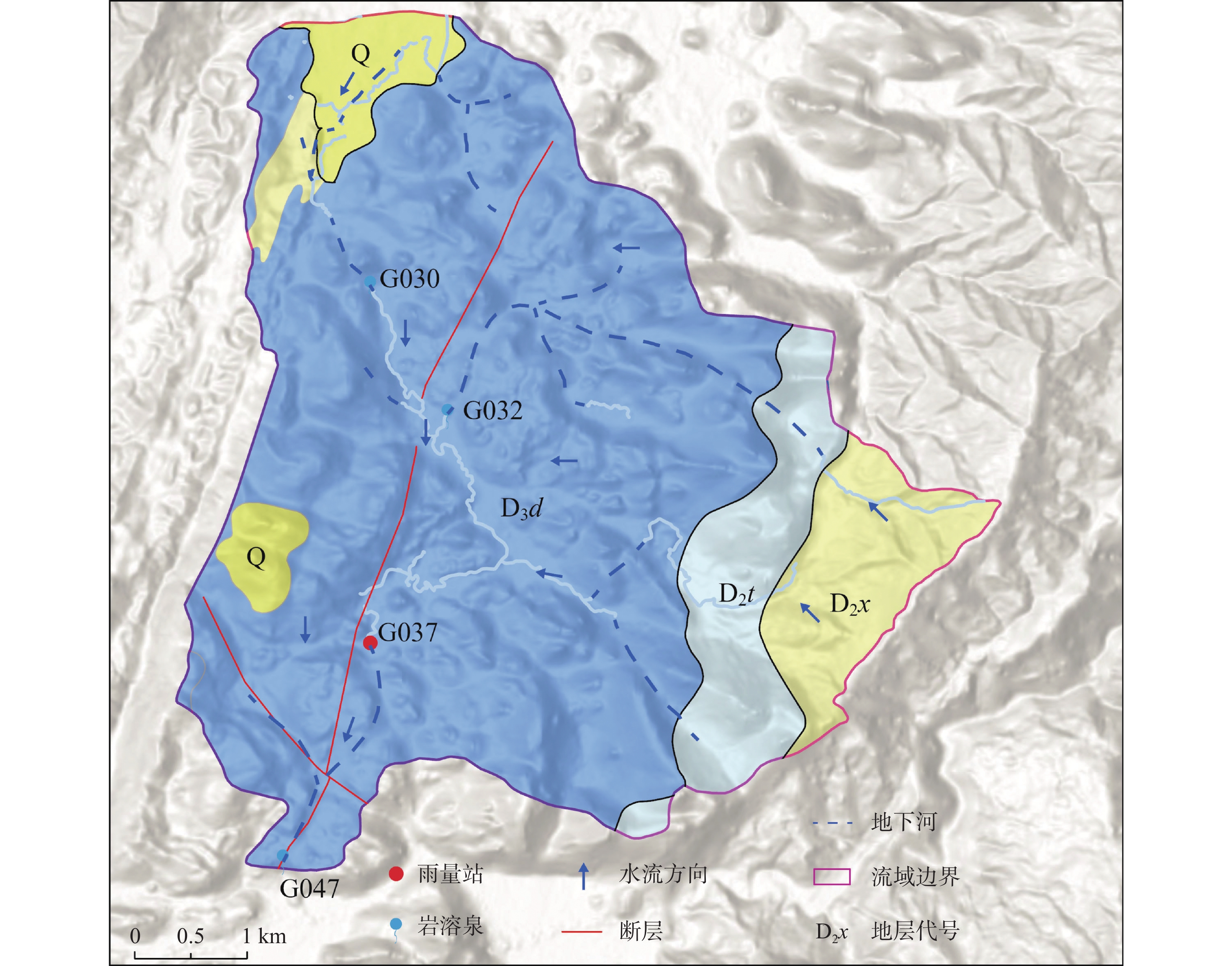
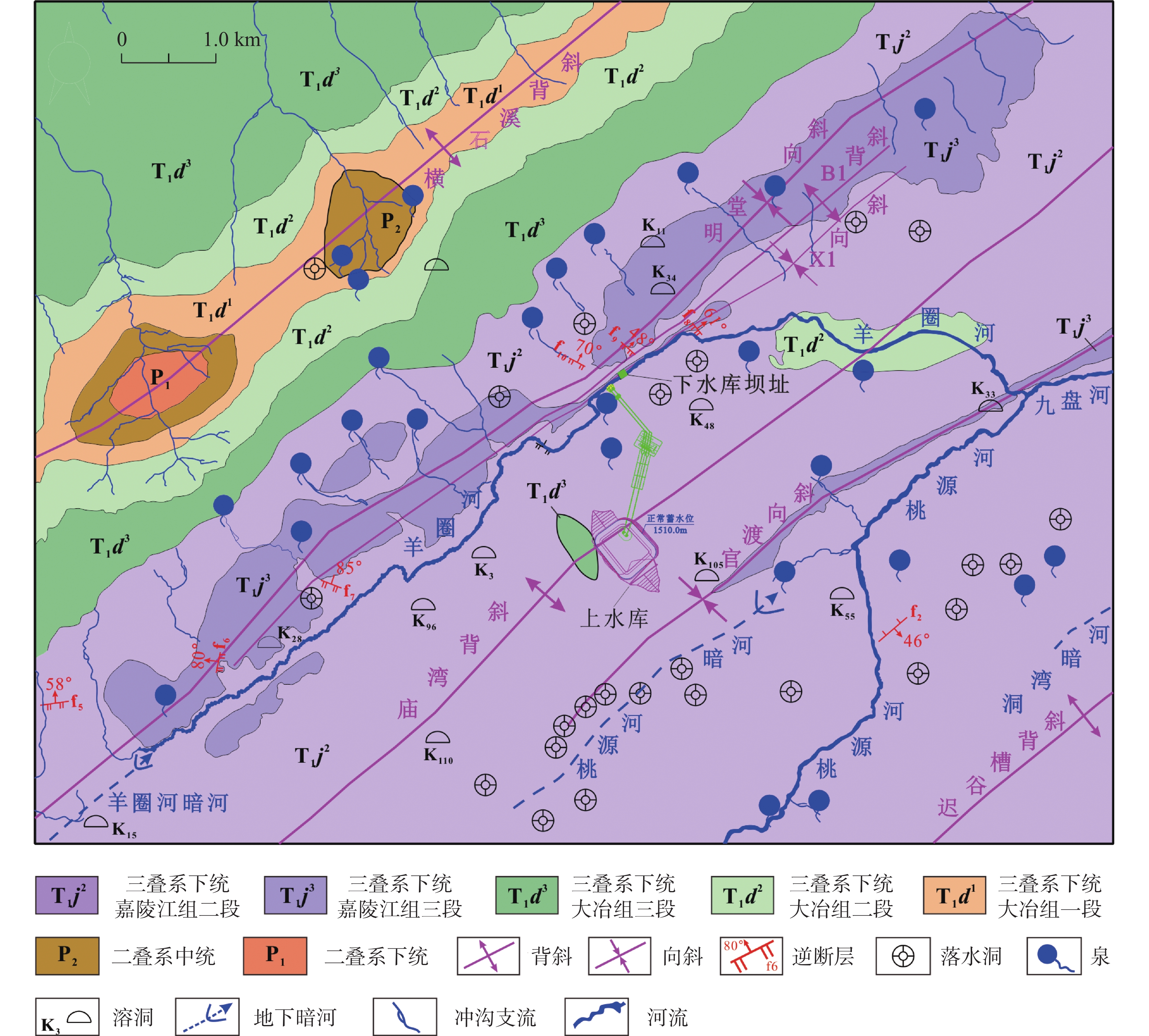
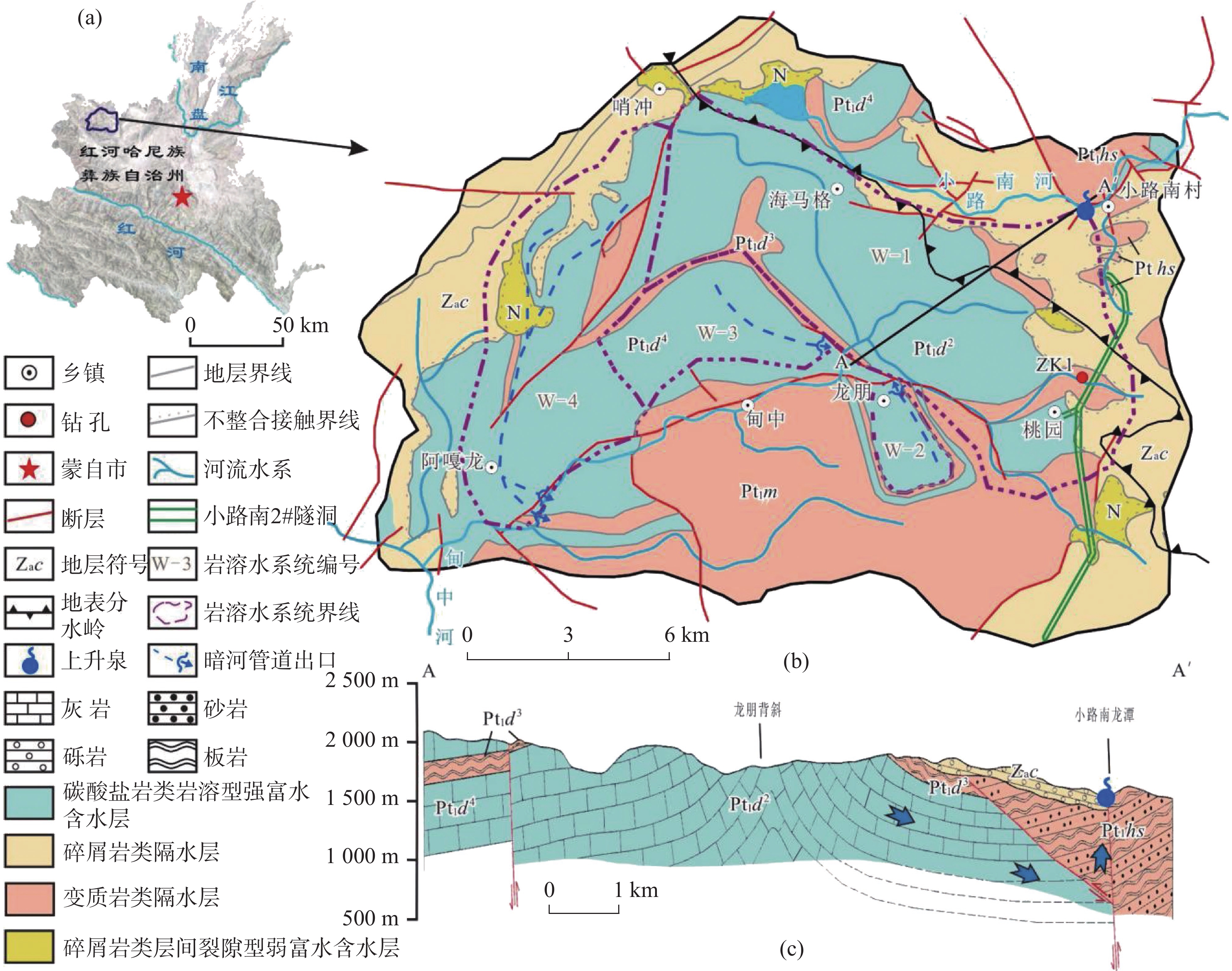
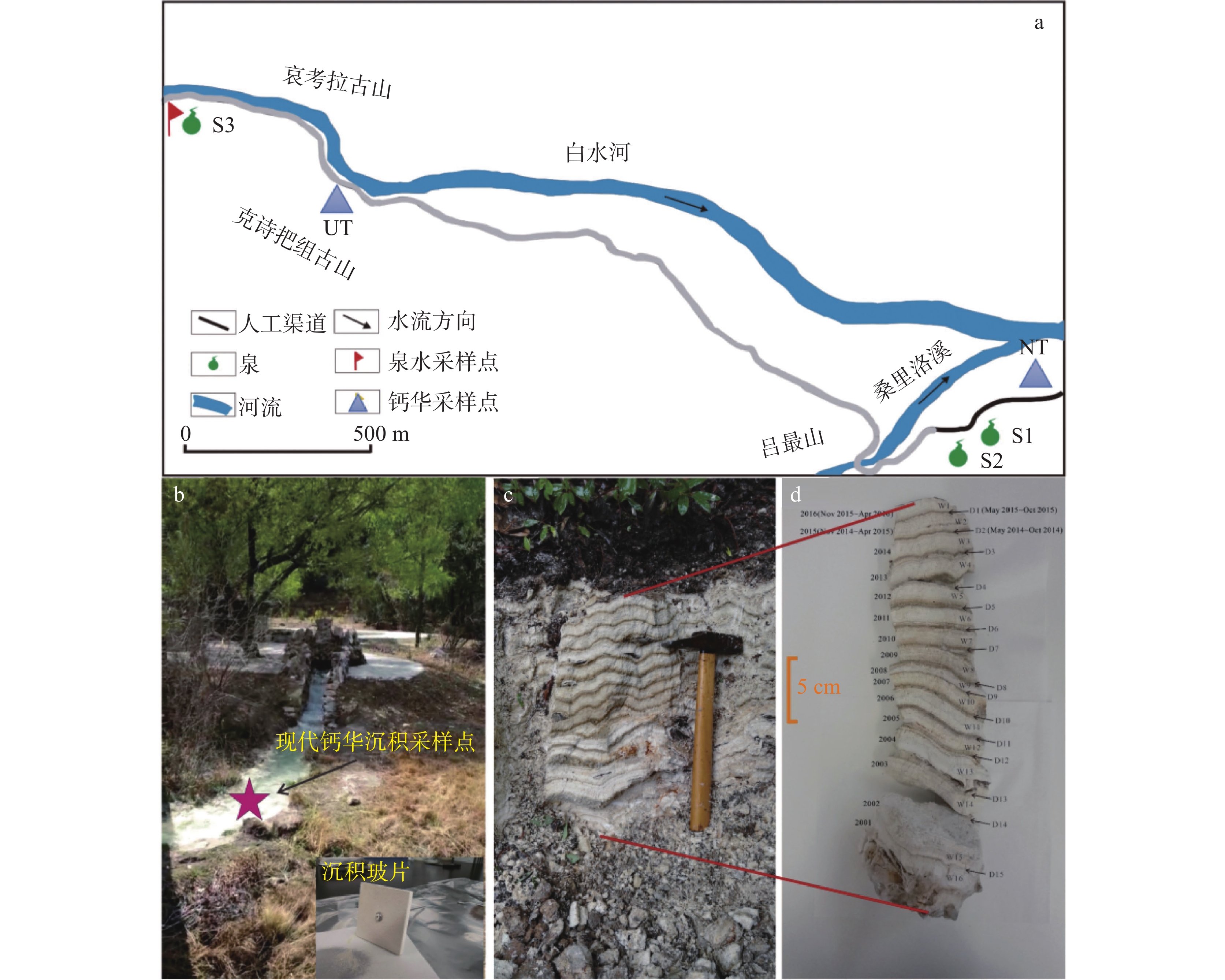
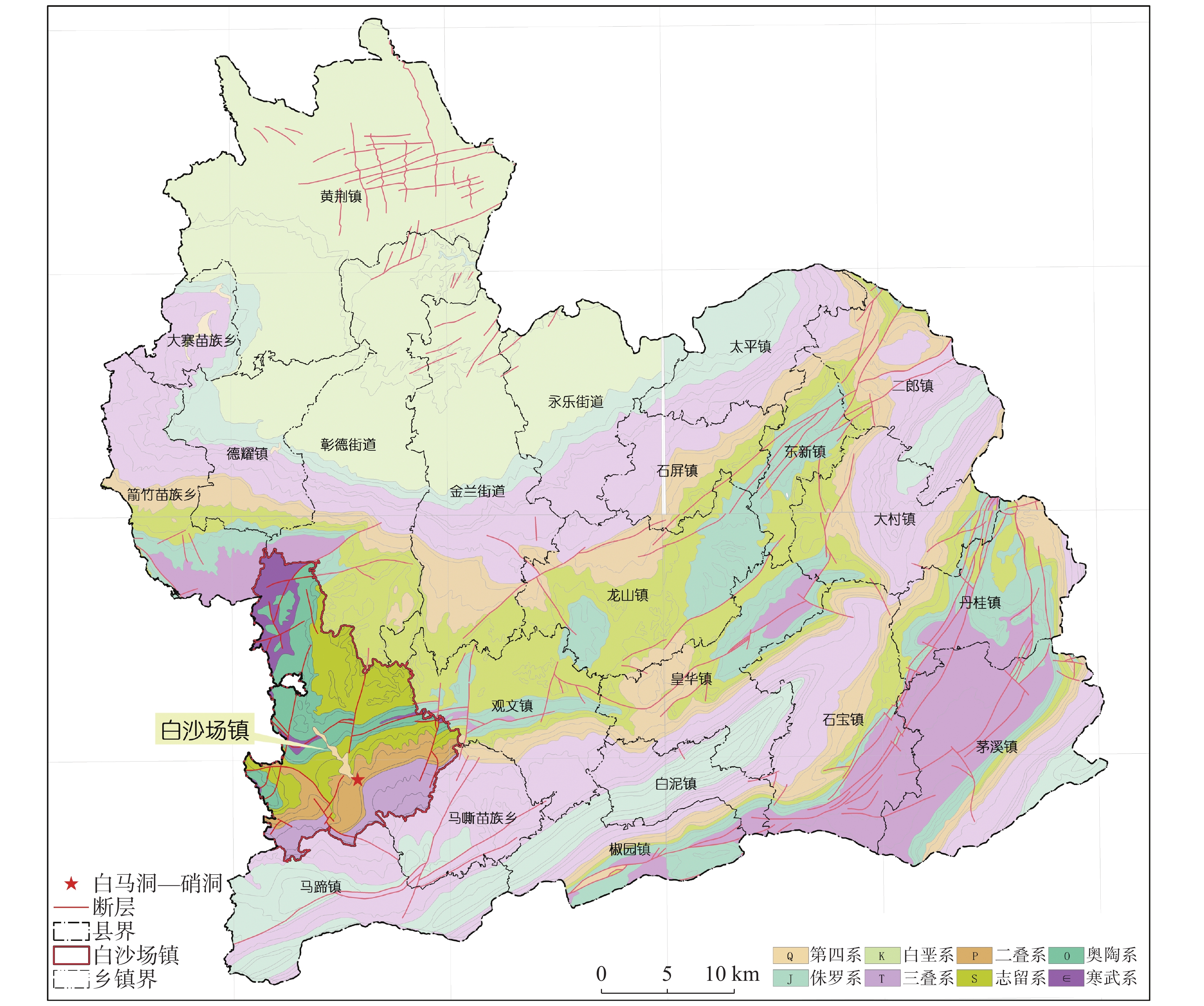
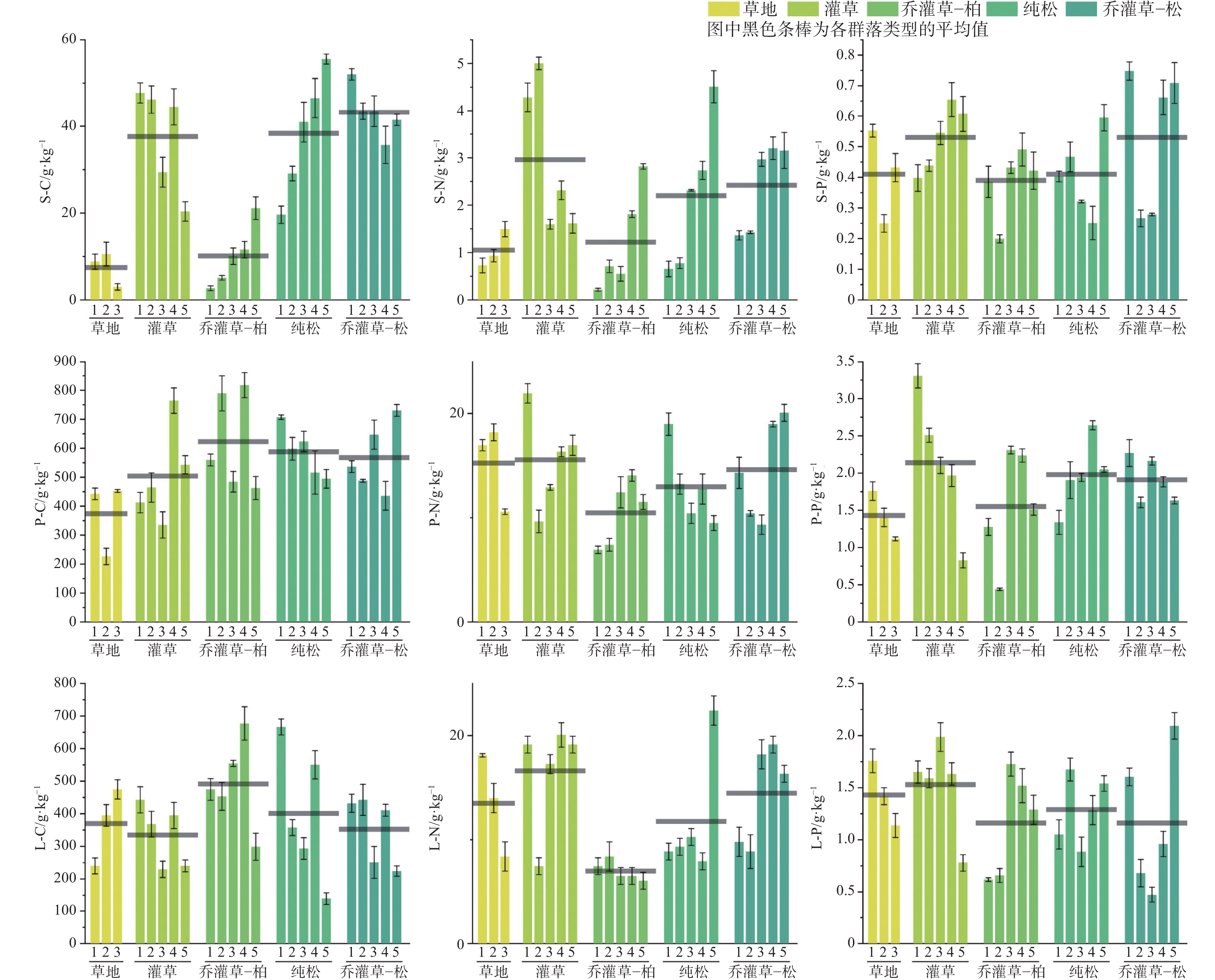
At present, China is deploying the basic survey of water resources. Provinces have initiated surveys of surface reserves and investigations into the quantity and quality of groundwater resources based on their specific circumstances. The goal is to accurately assess the surface water reserves as well as the quantity and quality of groundwater resources within each province. Over the years, Yunnan Province has carried out small-scale evaluation work, including regional hydrogeological surveys at scales of 1:200,000 and 1:250,000, county-level hydrogeological surveys at 1:100,000, and hydrogeological environment geological surveys at key river basins at 1:50,000. Since the 1980s, specialized groundwater dynamic monitoring has been initiated, and the construction of the national groundwater monitoring project has been launched in 2017. Based on extensive investigations, explorations, and thematic research findings, this paper comprehensively discusses and evaluates the current status of exploitation and utilization of groundwater, mineral water, and geothermal water in Yunnan Province through summary, statistical analysis, and comprehensive evaluation. The evaluation results show that Yunnan's distinctive plateau landforms, stratigraphic structures, neotectonic movements, and hydrogeological conditions have contributed to abundant groundwater, mineral water, and geothermal resources. On the basis of regional hydrogeological investigations and evaluations, the natural groundwater resources in Yunnan were estimated using the multi-year average underground runoff modulus method, yielding a total of 75.244 billion m3·a−1. The recoverable resources amount to 19.035 billion m3·a−1. Mineral water resources are generally underexplored, making accurate resource quantification challenging. The evaluation uses summaries of spring flow during the dry season and calculations of water inflow from drilling and pumping tests. The total mineral water resources is estimated at 154.37×104 m3·d−1, with a recoverable volume of 123.50×104 m3·d−1. Yunnan mainly utilizes natural hot springs and geothermal fluids extracted by artificial drilling. The province is rich in geothermal resources, with numerous hot springs distributed across almost all counties and cities. There are 851 naturally exposed hot springs with temperatures of 25°C or higher. Calculation results of geothermal resources through the heat storage method indicate that the heat flow is 9,216.3 L·s−1, and the natural heat release is 1,056,443.27 kJ·s−1. The reserves of geothermal resources amount to 197.77×1015 kJ. This is equivalent to 6,748.50×106 t of standard coal. The mining heat is 435,116.5×108 kJ·a−1, with an additional 120,377×108 kJ·a−1 extracted. The development and utilization rate is about 27.66%. According to the monitoring results of groundwater, the overall water quality is high. Compared to other regions across Yunnan Province, the proportion of water classified as Class I–III is slightly higher. The quality of geothermal water is superior to that of pore water, which in turn is better than that of bedrock water. Groundwater quality in the red bed area of bedrock water is more complex and variable. The water quality of the weathered fissure water in the red bed area is generally higher in the mountainous and hilly areas. In low-lying areas such as the intermountain basins and valley bottoms, water quality is highly variable and often poor due to the slow water circulation. For example, the third member of the Jiangdihe Formation (K2j3) of the Upper Cretaceous in Middle Yunnan contains gypsum and rock salt, and well-developed dissolution fissures facilitate groundwater accumulation. When recharge conditions are favorable, groundwater circulation is rapid, and drainage is efficient, resulting in generally high water quality. However, in areas with slow groundwater runoff, chloride and sulfate concentrations often exceed the permitted levels significantly, leading to poor water quality. Mineral water is classified into five categories: metasilicic mineral water, carbonated mineral water, trace element mineral water, salt mineral water, and compound mineral water. There are 24 hydrochemical types of geothermal fluids, including HCO3-Ca, HCO3-Na, HCO3-Ca·Na, HCO3-Ca·Mg, HCO3·SO4-Ca, HCO3·SO4-Na, and HCO3·SO4- Ca·Na, etc.This paper also summarizes and analyzes the existing issues and deficiencies in current investigations and evaluations of water resources, such as low overall investigation accuracy, insufficient systematic consideration of water system delineation, limited parameters for groundwater resources, and a lack of integrated systems for the investigation, evaluation, development, utilization, and management of geothermal resources. For the future investigations of groundwater, mineral water, and geothermal water, it is suggested that a comprehensive application of field investigations, drilling, geophysical exploration, tracing, and other technical methods, combined with advanced techniques such as hydrodynamics, hydrochemistry, environmental isotope analysis, and modeling, should be used to scientifically delineate the boundaries of water systems in karst areas. Pilot work and scientific research should be conducted in small river basins to address critical parameters in groundwater evaluation. Research should focus on water balance in small watersheds and the processes and mechanisms of water transformation among precipitation, surface water, and ground water. The groundwater monitoring network in Yunnan Province should be improved by leveraging the National Groundwater Monitoring Project (Phase II) to complete monitoring of major basins. Monitoring stations should be strategically established in areas with significant geological issues in karst environments, ecologically sensitive and fragile zones, nature reserves, important wetlands, geothermal water-rich areas, and mineral water sources, thereby strengthening multi-dimensional systematic monitoring. Establishing a comprehensive water resources monitoring system will enhance the accuracy of groundwater resource evaluations and support the rational development and sustainable utilization of groundwater resources.

 Wechat
Wechat