Genesis mechanism of karst water revealed by hydrochemistry and isotopes in Pingliang, Gansu
-
摘要: 岩溶水是平凉地区的重要水资源之一。探明岩溶水的形成机制对于该地区岩溶水资源的可持续利用、水源地保护以及环境保护措施的制定具有重要意义,为推动区域水资源的可持续发展和水环境健康提供了科学依据和理论支持。岩溶水的水化学成分可以揭示补给、径流和排泄过程,而同位素示踪方法则有助于指示岩溶水系统的范围和动态变化。因此,本研究通过水化学和同位素分析阐明了平凉地区岩溶水的成因机制。研究结果表明,该地区地下水主要分为HCO3-Ca型和HCO3·SO4-Na型两类。Spearman相关分析和离子比例系数分析表明,白云石、方解石和蒸发盐主导了平凉岩溶水的水化学形成。水文地质条件及氢氧同位素结果进一步证实,岩溶水主要来源于大气降水补给。基于岩溶水成因模型的识别,确定平凉地区存在两种岩溶水成因模式。尽管两者均接受来自山前的大气降水补给,并通过岩溶裂隙流经上覆地层进入含水层,但由于上覆地层的差异,导致水化学特征的不同。这表明,上覆地层中矿物溶解作用是平凉岩溶水化学成分形成的主要因素。Abstract:
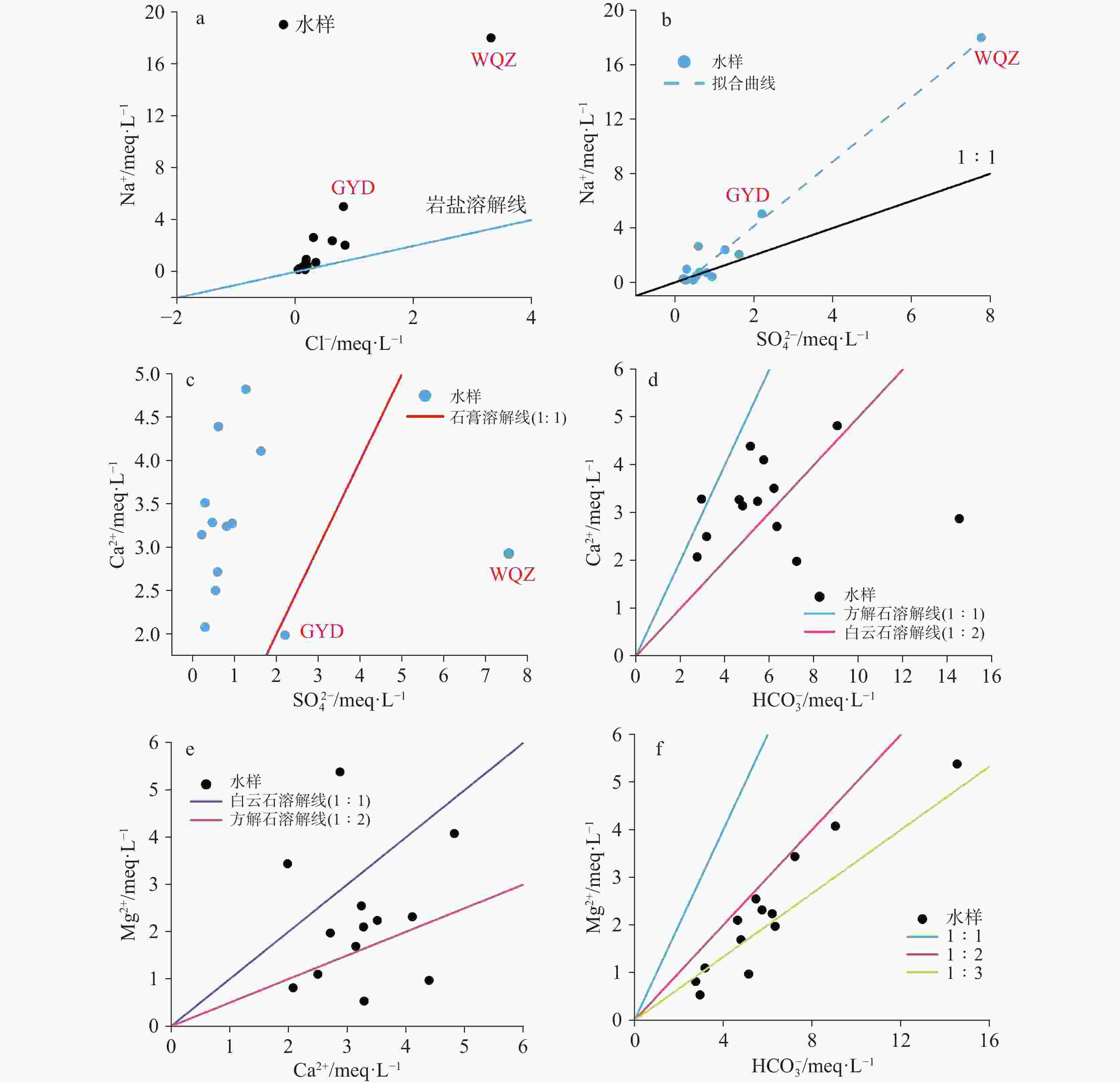
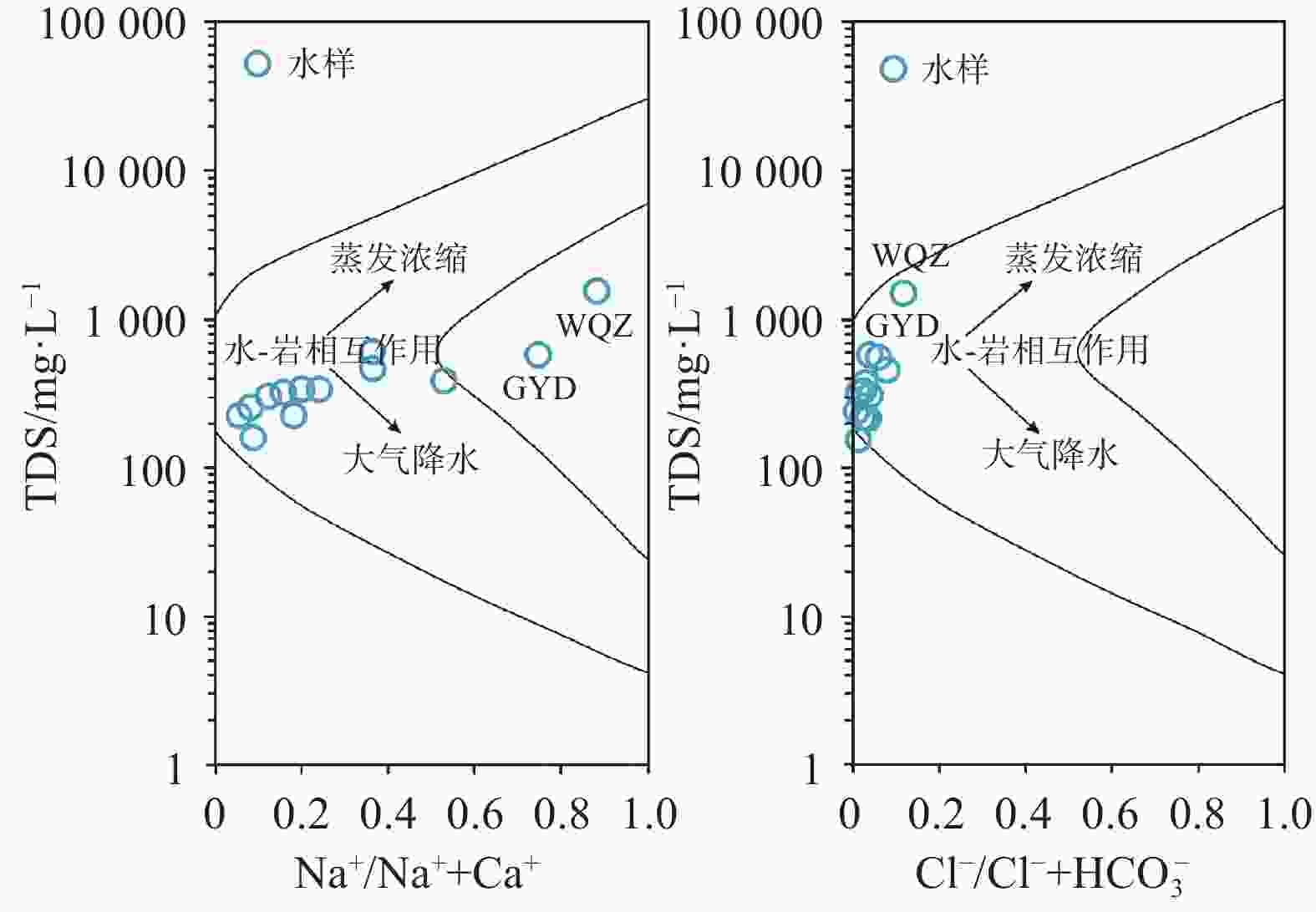
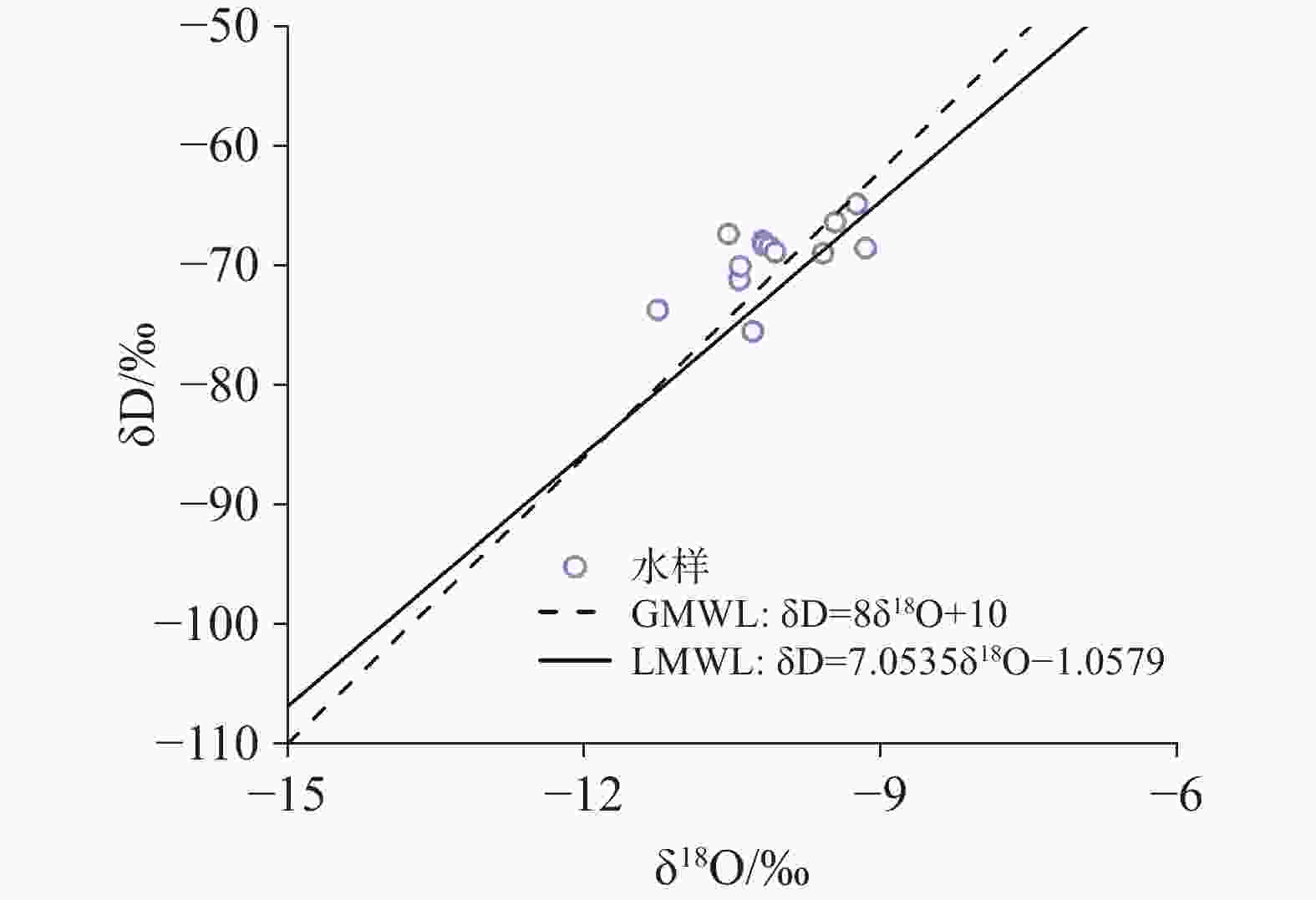
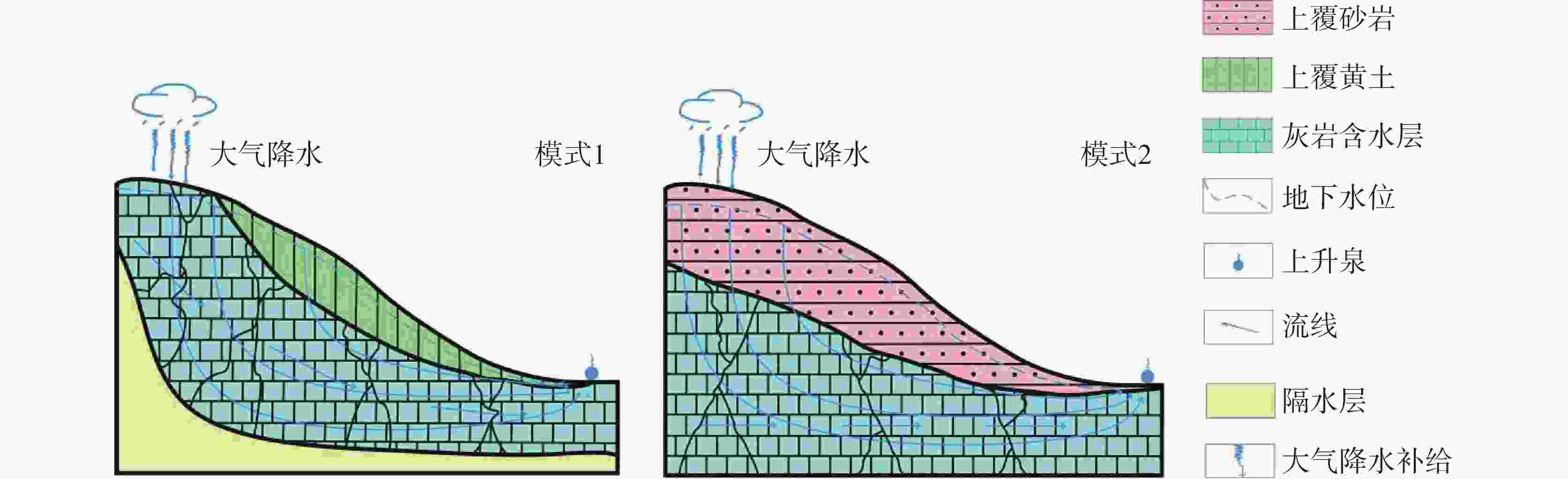
Karst water is a significant water resource in the Pingliang region. Investigating the formation mechanism of karst water is crucial for the sustainable utilization of karst water resources, the protection of water sources, and the formulation of environmental protection measures in the area. Such studies provide a scientific basis and theoretical support for promoting the sustainable development of regional water resources and maintaining the health of the water environment. The hydrochemical composition of karst water can reveal the processes of recharge, runoff, and discharge, while isotopic tracing methods help delineate the extent and dynamic changes within the karst water system. To uncover the genesis mechanism of karst water in Pingliang City, we conducted a comprehensive analysis of its hydrochemical characteristics and sources using methods such as Piper trilinear diagrams, mathematical modeling, and ion ratio coefficients. The results indicate that the groundwater in the region can be mainly classified into two types: HCO3-Ca and HCO3·SO4-Na, with Ca2+ and Na+ as the dominant cations and ${\rm{HCO}}_3^{-}$ as the primary anion. The pH value ranges from 6.72 to 6.90, exhibiting overall weakly alkaline hydrochemical characteristics. Total Dissolved Solids (TDS) concentrations in the groundwater range from 158.01 mg·L−1 to 1,519.40 mg·L−1. Spearman correlation analysis shows that the hydrochemistry of karst water in the study area is primarily controlled by the dissolution of minerals such as dolomite and evaporites. TDS is highly positively correlated with the contents of Na+, Mg2+, Cl−, ${\rm{SO}}_4^{2-}$ and ${\rm{HCO}}_3^{-}$. Additionally, the content of Mg2+ is significantly positively correlated with the contents of the main anions Cl−, ${\rm{SO}}_4^{2-}$ and ${\rm{HCO}}_3^{-}$ (r > 0.70, p < 0.01), and the content of Na+ also shows a significant positive correlation with the contents of Cl− and ${\rm{SO}}_4^{2-}$ (r > 0.98, p < 0.01). In the analysis of ion proportional coefficients, the hydrochemical characteristics of karst water in Pingliang City are mainly influenced by rock weathering. Na+ not only originates from the dissolution of rock salt, but may also come from the dissolution of sulfate or silicate minerals. Mg2+ is derived from the combined action of calcite and dolomite. Ca2+ is produced not only from the dissolution of dolomite but also from the dissolution of calcite and dolomite. During the rock weathering process, dolomite, calcite, and evaporites dominate the hydrochemical formation of karst water in Pingliang City. The Gibbs diagram combined with the analysis of mineral saturation indices further confirms that the dissolution of evaporites, carbonates, and silicate minerals is the natural source of hydrochemical components in Pingliang City. Ca2+, Mg2+ and ${\rm{HCO}}_3^{-}$ in the karst groundwater of Pingliang City mainly originate from the combined dissolution of calcite and dolomite. Hydrogeological conditions and hydrogen-oxygen isotope analyses show that δD values range from -75.45‰ to -64.80‰, with an average of -69.20‰; while δ18O values range from -11.26‰ to -9.16‰, averaging -10.07‰. These results indicate that all groundwater is recharged by infiltration of atmospheric precipitation.Based on the identification of the gensis model for karst water, it has been determined that there are two genesis modes of karst water in the Pingliang region. Mode 1: Atmospheric precipitation infiltrates through the overlying Quaternary loess layer, continuously dissolving sulfate minerals within the loess, and enters the limestone confined aquifer through karst fissures formed by faults, eventually emerging as springs at the interface between the loess and limestone aquifer. In this mode, the chemical type of karst water is mainly HCO3·SO4-Na. Mode 2: Atmospheric precipitation infiltrates through the overlying Quaternary sandstone layer, continuously dissolving carbonate minerals in the sandstone layer, and enters the limestone confined aquifer through karst fissures formed by faults, eventually emerging as springs at the interface between the sandstone and limestone aquifers. In this mode, the chemical composition of karst water is mainly HCO3-Ca. Although both modes receive atmospheric precipitation recharge from the windward side of the mountain and enter the aquifer through karst fissures in the overlying strata, differences in these strata result in distinct hydrochemical characteristics. This indicates that the mineral dissolution in the overlying strata is the main factor influencing the hydrochemical composition of karst water in Pingliang.-
Key words:
- hydrochemistry /
- isotope /
- genesis mechanism /
- karst water /
- the Pingliang region
-
表 1 样品的水化学和同位素结果(mg∙L−1)
Table 1. Hydrochemical and isotope results of samples (mg∙L−1)
样品
编号上覆地
层岩性采样
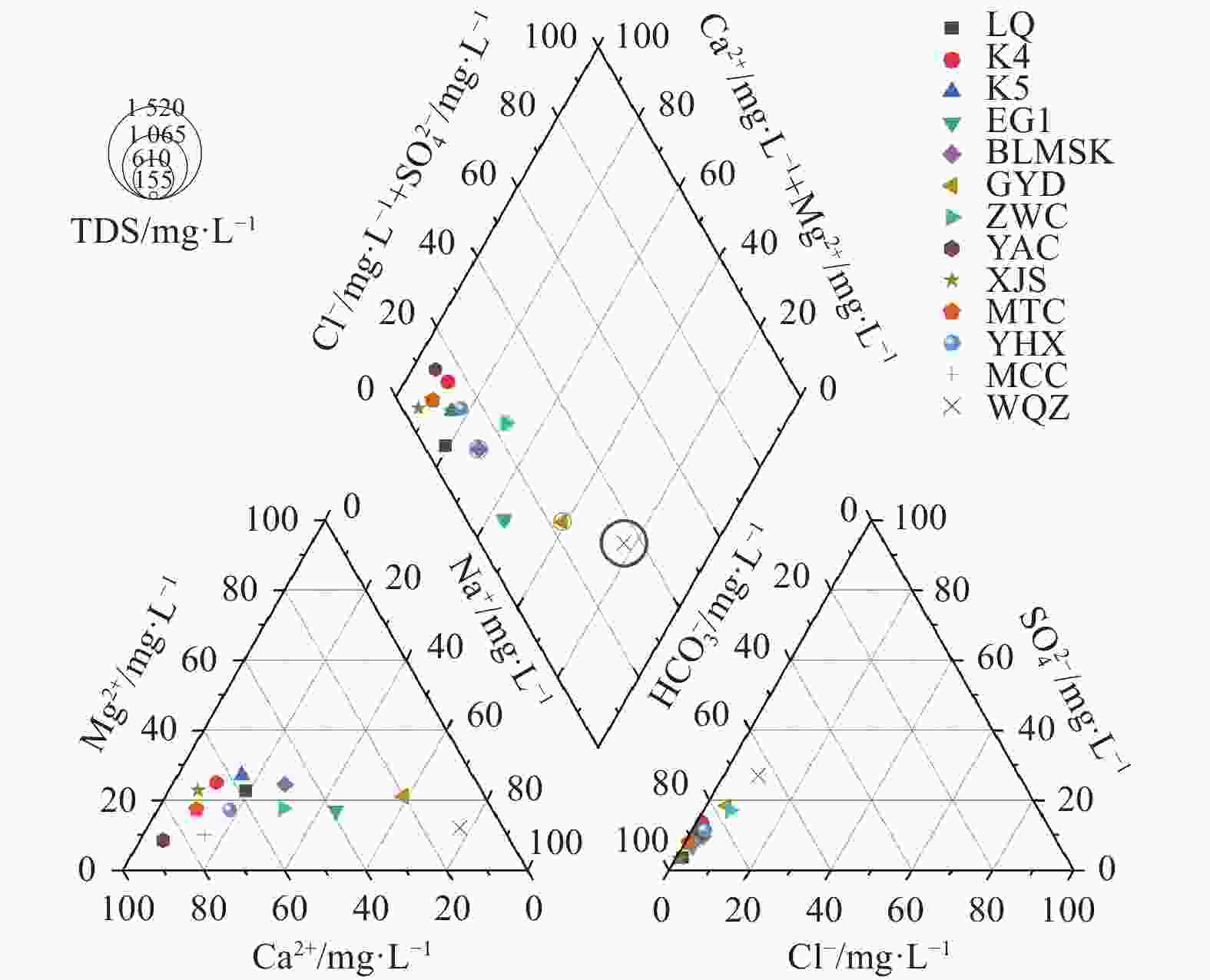
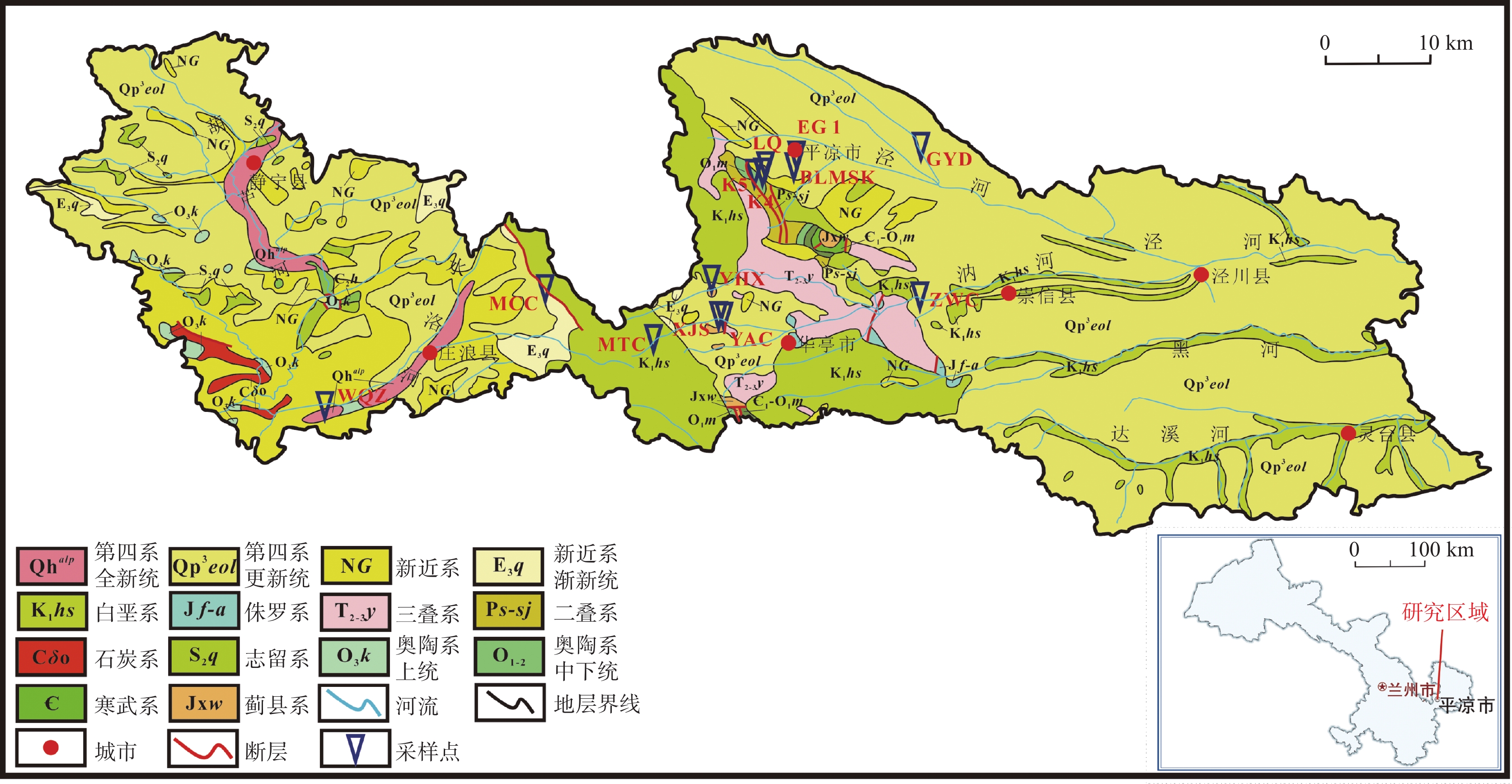
日期pH Ca2+ Mg2+ Na+ K+ Cl− ${\rm{SO}}_4^{2-}$ Ba2+ ${\rm{NO}}_3^{-}$ ${\rm{HCO}}_3^{-}$ TDS δ18OV-SMOW δDV-SMOW 水质类型 mg·L−1 ‰ LQ 砂岩 2024.07.24 7.17 70.34 27.17 22.23 0.89 6.66 14.24 0.051 3.55 378.79 334.85 −10.43 −71.16 HCO3-Ca K4 砂岩 2024.07.24 7.68 65.63 25.56 9.35 1.47 4.76 45.28 0.043 7.69 283.41 301.60 −11.26 −73.67 HCO3-Ca K5 砂岩 2024.07.24 7.42 64.94 30.93 16.25 1.85 6.40 38.92 0.018 6.66 333.69 332.98 −10.19 −67.88 HCO3-Ca EG1 砂岩 2024.07.24 7.44 54.39 23.95 60.72 1.19 10.89 28.49 0.036 7.69 386.87 381.12 −9.59 −68.95 HCO3-Ca BLMSK 砂岩 2024.07.25 7.22 96.59 49.50 55.12 1.32 22.26 60.93 0.048 25.60 552.21 587.89 −9.16 −68.51 HCO3-Ca GYD 黄土 2024.07.25 7.58 39.81 41.77 115.47 1.39 28.97 105.78 0.013 14.61 440.85 569.09 −10.29 −75.45 HCO3·SO4-Na ZWC 砂岩 2024.07.25 7.41 82.30 28.15 47.13 2.24 29.95 78.18 0.040 20.45 350.54 464.01 −9.46 −66.32 HCO3-Ca YAC 砂岩 2024.07.26 7.46 65.88 6.48 3.97 0.48 6.00 22.59 0.012 25.61 180.39 221.37 −10.42 −70.03 HCO3-Ca XJS 砂岩 2024.07.26 7.44 63.00 20.54 5.80 0.67 2.53 10.05 0.027 2.38 293.01 251.65 −10.18 −68.25 HCO3-Ca MTC 砂岩 2024.07.26 8.20 41.64 9.90 4.10 1.32 1.83 14.19 0.029 0.95 168.13 158.01 −10.54 −67.33 HCO3-Ca YHX 砂岩 2024.07.26 8.15 50.12 13.35 11.19 2.51 7.17 26.09 0.035 14.67 194.09 222.23 −10.12 −68.41 HCO3-Ca MCC 砂岩 2024.07.26 7.39 88.00 11.79 16.63 1.26 12.38 29.40 0.042 1.48 314.09 318.05 −10.07 −68.85 HCO3-Ca WQZ 黄土 2024.07.26 7.57 57.67 65.39 413.73 2.83 117.36 372.71 0.084 45.28 886.84 1519.40 −9.24 −64.80 HCO3·SO4-Na 表 2 水化学指标Spearman相关系数矩阵
Table 2. Spearman correlation coefficient matrix of hydrochemical indicators
pH Ca2+ Mg2+ Na+ K+ Ba2+ Si2+ Cl− ${\rm{SO}}_4^{2-}$ F− ${\rm{NO}}_3^{-}$ ${\rm{HCO}}_3^{-}$ TDS pH 皮
尔
逊
相
关
性1 Ca2+ −0.672* 1 Mg2+ −0.338 0.105 1 Na+ −0.054 −0.156 0.801** 1 K+ 0.351 −0.110 0.483 0.582* 1 Ba2+ −0.137 0.280 0.597* 0.691** 0.520 1 Si2+ −0.368 0.321 0.338 0.195 0.232 0.384 1 Cl− −0.080 −0.035 0.792** 0.984** 0.635* 0.721** 0.208 1 ${\rm{SO}}_4^{2-}$ −0.027 −0.105 0.797** 0.986** 0.642* 0.687** 0.134 0.991** 1 F− −0.271 −0.162 0.874** 0.854** 0.377 0.441 0.358 0.819** 0.819** 1 NO3- −0.107 0.107 0.660* 0.770** 0.502 0.487 0.020 0.818** 0.804** 0.686** 1 ${\rm{HCO}}_3^{-}$ −0.368 0.148 0.940** 0.906** 0.469 0.741** 0.396 0.899** 0.883** 0.868** 0.706** 1 TDS −0.183 0.015 0.876** 0.981** 0.582* 0.733** 0.257 0.984** 0.978** 0.864** 0.803** 0.960** 1 *. 在 0.05 级别(双尾),相关性显著。**. 在 0.01 级别(双尾),相关性显著。 表 3 样品水化学指示的矿物溶解饱和指数
Table 3. Indices of mineral dissolution saturation indicated by sample hydrochemistry
样品编号 方解石 白云石 石膏 岩盐 LQ 0.18 0.31 −2.48 −8.40 K4 0.54 1.02 −1.99 −8.92 K5 0.34 0.71 −2.08 −8.55 EG1 0.34 0.68 −2.28 −7.75 BLMSK 0.47 0.99 −1.83 −7.50 GYD 0.35 1.07 −1.92 −7.06 ZWC 0.42 0.73 −1.71 −7.43 YAC 0.18 −0.31 −2.2 −9.18 XJS 0.32 0.51 −2.63 −9.40 MTC 0.68 1.1 −2.57 −9.67 YHX 0.75 1.29 −2.27 −8.65 MCC 0.43 0.33 −2.05 −8.25 WQZ 0.66 1.71 −1.43 −5.95 -
[1] Jiang Y J, Wu Y X, Groves C, Yuan D X, Kambesis P. Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China[J]. Journal of Contaminant Hydrology, 2009, 109(1): 49-61. [2] 王楠, 胥芹, 孙小艳, 武显仓, 李常锁, 高帅. 趵突泉泉域岩溶水化学特征及成因研究[J]. 中国岩溶, 2024, 43(2): 279-290. doi: 10.11932/karst20240203WANG Nan, XU Qin, SUN Xiaoyan, WU Xiancang, LI Changsuo, GAO Shuai. Hydrochemical characteristics and formation mechanism of karst water in Baotu Spring watershed[J]. Carsologica Sinica, 2024, 43(2): 279-290. doi: 10.11932/karst20240203 [3] Nico G, Chen Z, Auler A S, Bakalowicz M, Broda S, Drew D, Hartmann J, Jiang G H, Moosdorf N, Stevanovic Z, Veni G. Global distribution of carbonate rocks and karst water resources[J]. Hydrogeology Journal, 2020, 28(5): 1661-1677. doi: 10.1007/s10040-020-02139-5 [4] Zoran S. Global distribution and use of water from karst aquifers[J]. Geological Society, London, Special Publications, 2018, 466(1): 217-236. doi: 10.1144/SP466.17 [5] 梁永平, 赵春红. 中国北方岩溶水功能[J]. 中国矿业, 2018, 27(S2): 297-299. doi: 10.12075/j.issn.1004-4051.2018.S2.078LIANG Yongping, ZHAO Chunhong. Karst water function in Northern China[J]. China Mining Magazine, 2018, 27(S2): 297-299. doi: 10.12075/j.issn.1004-4051.2018.S2.078 [6] 刘燚, 康凤新, 张文强, 许庆宇, 秦鹏, 赵强, 李嘉龙, 崔洋, 隋海波, 郑婷婷. 济南长孝岩溶水系统地下水富集区补给源识别及其成因机制[J]. 地质科技通报, 2024, 43(6): 292-305.LIU Yi, KANG Fengxin, ZHANG Wenqiang, XU Qingyu, QIN Peng, ZHAO Qiang, LI Jialong, CUI Yang, SUI Haibo, ZHENG Tingting. Identification and genetic mechanism of recharge sources in groundwater-rich area of Changxiao karst water system in Jinan City [J]. Bulletin of Geological Science and Technology, 2024, 43(6): 292-305. [7] 马国哲. 平凉市灰岩岩溶水赋存规律探讨[J]. 甘肃地质学报, 2001(1): 63-68.MA Guozhe. Ingury into the hosting regularity of karstic water of limestonge in urban district of Pingliang[J]. Acta Geologica Gansu, 2001(1): 63-68. [8] 中国地质学会岩溶地质专业委员会. 中国北方岩溶和岩溶水[M]. 北京: 地质出版社, 1982.Karst Geology Professional Committee of the Geological Society of China. Karst and karst water in Northern China [M]. Beijing: Geology Press, 1982. [9] 刘春蓁, 刘志雨, 谢正辉. 地下水对气候变化的敏感性研究进展[J]. 水文, 2007(2): 1-6. doi: 10.3969/j.issn.1000-0852.2007.02.001LIU Chunzhen, LIU Zhiyu, XIE Zhenghui. Recent advances in esearch on sensitivity of groundwater to climate changes[J]. Journal of China Hydrology, 2007(2): 1-6. doi: 10.3969/j.issn.1000-0852.2007.02.001 [10] Wang J Z, Wu J L, Jia H J. Analysis of spatial variation of soil salinization using a hydrochemical and stable isotopic method in a semiarid irrigated basin, Hetao Plain, Inner Mongolia, North China[J]. Environmental Processes, 2016, 3(4): 723-733. doi: 10.1007/s40710-016-0179-6 [11] Liu Y P, Yamanaka T. Tracing groundwater recharge sources in a mountain−plain transitional area using stable isotopes and hydrochemistry[J]. Journal of Hydrology, 2012, 464: 116-126. [12] Željka B, Briški M, Marković T. Use of hydrochemistry and isotopes for improving the knowledge of groundwater flow in a semiconfined aquifer system of the Eastern Slavonia (Croatia)[J]. Catena, 2016, 142: 153-165. doi: 10.1016/j.catena.2016.03.010 [13] Amor B M, Mzali H, Zouari K, Hezzi, H. Hydrochemical and isotopic assessment of groundwater quality in the Quaternary shallow aquifer, Tazoghrane region, north-eastern Tunisia[J]. Quaternary International, 2014, 338: 51-58. doi: 10.1016/j.quaint.2014.01.023 [14] 贾超, 王丛, 刘森, 杨霄, 刘文, 高帅, 朱恒华. 济南西部冲积平原地下水水文地球化学特征研究[J]. 水利水电技术(中英文), 2022, 53(3): 49-60.JIA Chao, WANG Cong, LIU Sen, YANG Xiao, LIU Wen, GAO Shuai, ZHU Henghua. Study on hydrogeochemical characteristics of groundwater in the alluvial plain of western Jinan[J]. Water Resources and Hydropower Engineering, 2022, 53(3): 49-60. [15] 张雅, 苏春利, 马燕华, 刘伟江. 水化学和环境同位素对济南东源饮用水源地地下水演化过程的指示[J]. 环境科学, 2019, 40(6): 2667-2674.ZHANG Ya, SU Chunli, MA Yanhua, LIU Weijiang. Indicators of groundwater evolution processes based on hydrochemistry and environmental isotopes: A case study of the dongyuan drinking water source area in Ji'nan city[J]. Environmental Science, 2019, 40(6): 2667-2674. [16] 高宗军, 徐军祥, 王世臣, 李常锁, 韩克, 李佳佳, 罗斐, 马河宽. 济南岩溶水微量元素分布特征及其水文地质意义[J]. 地学前缘, 2014, 21(4): 135-146.GAO Zongjun, XU Junxiang, WANG Shichen, LI Changsuo, HAN Ke, LI Jiajia, LUO Fei, MA Hekuan. The distribution characteristics and hydrogeological significance of trace elements in karst water, Jinan, China[J]. Earth Science Frontiers, 2014, 21(4): 135-146. [17] Sun Z Y, Ma R, Wang Y X, Ma, T, Liu, Y D. Using isotopic, hydrogeochemical-tracer and temperature data to characterize recharge and flow paths in a complex karst groundwater flow system in Northern China[J]. Hydrogeology Journal, 2016, 24(6): 1393. doi: 10.1007/s10040-016-1390-2 [18] Chen X Q, Han C H, Li S X, Wang Z Z, Liu D, Guan Q H, Zhang W J. Exploring the hydrogeochemical formation and evolution of the karst aquifer system in the Yufu River based on hydrochemistry and isotopes[J]. Sustainability, 2024, 16(15): 6580. doi: 10.3390/su16156580 [19] Lu S S, Zhou N Q, Jiang S M, Zheng X Q. Combining hydrochemistry and environmental isotopes to study hydrogeochemical evolution of karst groundwater in the Jinci spring area, North China[J]. Carbonates and Evaporites, 2023, 38(2): 36. [20] 马致远. 环境同位素方法在平凉市岩溶地下水研究中的应用[J]. 地质论评, 2004(4): 433-439. doi: 10.3321/j.issn:0371-5736.2004.04.015MA Zhiyuan. Application of the environmental isotope technique to the study of karst groundwater in Pingliang City[J]. Geological Review, 2004(4): 433-439. doi: 10.3321/j.issn:0371-5736.2004.04.015 [21] 张彦林, 李生永, 付东林, 崔旭东. 陇东盆地西部岩溶地下水形成机制研究[J]. 中国地质, 2006, 33(6): 1393-1399. doi: 10.3969/j.issn.1000-3657.2006.06.024ZHANG Yanlin, LI Shengyong, FU Donglin, CUI Xudong. Formation mechanism of karst groundwater in the western Longdong basin, Northwestern China[J]. Geology in China, 2006, 33(6): 1393-1399. doi: 10.3969/j.issn.1000-3657.2006.06.024 [22] 马致远, 马蒂尔·亨德尔. 平凉隐伏岩溶水环境同位素研究[J]. 地球科学与环境学报, 2003(4): 60-66. doi: 10.3969/j.issn.1672-6561.2003.04.013MA Zhiyuan, Mathiel Handel. Environmental isotope study on groundwater in a covered ordovician carbonate rock of Pingliang, nw China[J]. Journal of Earth Sciences and Environment, 2003(4): 60-66. doi: 10.3969/j.issn.1672-6561.2003.04.013 [23] Jia H, Qian H, Zheng L, Feng W W, Wang H K, Gao Y Y. Alterations to groundwater chemistry due to modern water transfer for irrigation over decades[J]. Science of The Total Environment, 2020, 717: 137170. [24] 廖驾, 朱振华, 彭毅, 韦珊瑚, 罗朝晖, 刘状, 徐强强, 谢亘. 湘西北地区岩溶地下水水化学与氘氧同位素特征分析[J]. 中国岩溶, 2023, 42(3): 425-435, 481. doi: 10.11932/karst2023y003LIAO Jia, ZHU Zhenhua, PENG Yi, WEI Shanhu, LUO Zhaohui, LIU Zhuang, XU Qiangqiang, XIE Gen. Analysis on D/18O hydrochemical characterristics of karst groundwater in northwestern Hunan Province[J]. Carsologica Sinica, 2023, 42(3): 425-435, 481. doi: 10.11932/karst2023y003 [25] Jia H, Qu W G, Ren W H, Qian H. Impacts of chemical weathering and human perturbations on dissolved loads of the Wei River, the Yellow River catchment[J]. Journal of Hydrology, 2021, 603: 126950. doi: 10.1016/j.jhydrol.2021.126950 [26] 谢浩, 申豪勇, 张海岛, 林永生, 李军, 梁慧雅, 王志恒, 朱天龙, 邹胜章. 清江流域不同类型岩溶地下水化学分布特征及成因机制[J]. 环境科学, 2025, 46(3): 1417-1426.XIE Hao, SHEN Haoyong, ZHANG Haodao, LIN Yongsheng, LI Jun, LIANG Huiya, WANG Zhiheng, ZHU Tianlong, ZOU Shengzhang. Distributions and driving factors of hydrochemcial compositions in different types of karst groundwater in Qingjiang river basin [J]. Environmental Science, 2025, 46(3): 1417-1426. [27] 冯亚伟, 陈洪年, 卜华, 贾德旺. 羊庄岩溶水系统水化学成因及同位素特征[J]. 中国岩溶, 2019, 38(3): 394-403. doi: 10.11932/karst20190309FENG Yawei, CHEN Hongnian, BU Hua, JIA Dewang. Hydrochemical genesis and isotope characteristics of Yangzhuang karst water system[J]. Carsologica Sinica, 2019, 38(3): 394-403. doi: 10.11932/karst20190309 [28] Gibbs R J. Mechanisms controlling world water chemistry[J]. Science, 1970, 170(3962): 1088-1090. doi: 10.1126/science.170.3962.1088 [29] Marandi A, Paul S. Groundwater chemistry and the gibbs diagram[J]. Applied Geochemistry, 2018, 97: 209-212. doi: 10.1016/j.apgeochem.2018.07.009 [30] 姜凤, 周金龙, 周殷竹, 孙英, 韩双宝, 鲁涵. 巴伊盆地平原区地下水水化学特征及污染源识别[J]. 环境科学, 2023, 44(11): 6050-6061.JIANG Feng, ZHOU Jinlong, ZHOU Yinzhu, SUN Ying, HAN Shuangbao, LU Han. Hydrochemical characteristics and pollution source identification of groundwater in plain area of Barkol-Yiwu basin[J]. Environmental Science, 2023, 44(11): 6050-6061. [31] Aghazadeh N, Chitsazan M, Golestan Y. Hydrochemistry and quality assessment of groundwater in the Ardabil area, Iran[J]. Applied Water Science, 2017, 7(7): 3599-3616. doi: 10.1007/s13201-016-0498-9 [32] 仝晓霞, 刘存富. 西北干寒区冰雪融水氢氧同位素水文地质意义[J]. 环境科学与技术, 2018, 41(1): 57-63.TONG Xiaoxia, LIU Cunfu. Hydrogeological significance of hydrogen and oxygen isotopes in ice and snow melting water in northwest arid region[J]. Environmental Science & Technology, 2018, 41(1): 57-63 [33] Harmon C. Isotopic variations in meteoric waters[J]. Science, 1961, 133(3465): 1702-1703. doi: 10.1126/science.133.3465.1702 [34] 王恒纯. 同位素水文地质概论[M]. 北京: 地质出版社, 1991.WANG Hengchun. Introduction to Isotope Hydrogeology [M]. Beijing: Geology Press, 1991. [35] 喻生波. 平凉南部山区岩溶裂隙水分析[J]. 地下水, 2009, 31(4): 18-20. doi: 10.3969/j.issn.1004-1184.2009.04.006YU Shengbo. Analysis on karst groundwater in the montainous area of southern Pingliang[J]. Groundwater, 2009, 31(4): 18-20. doi: 10.3969/j.issn.1004-1184.2009.04.006 [36] 刘亚平. 山前平原地下水的补给特征及其影响因素[D]. 北京: 中国地质大学(北京), 2012.LIU Yaping. Characteristics of groundwater recharge in piedmont plains and its potential impact factors [D]. Beijing: China University of Geosciences (Beijing), 2012. -




 下载:
下载: