Dissolution process of carbonate rocks by different types of water in atmospheric environment and δ13C evolution of dissolved inorganic carbon
-
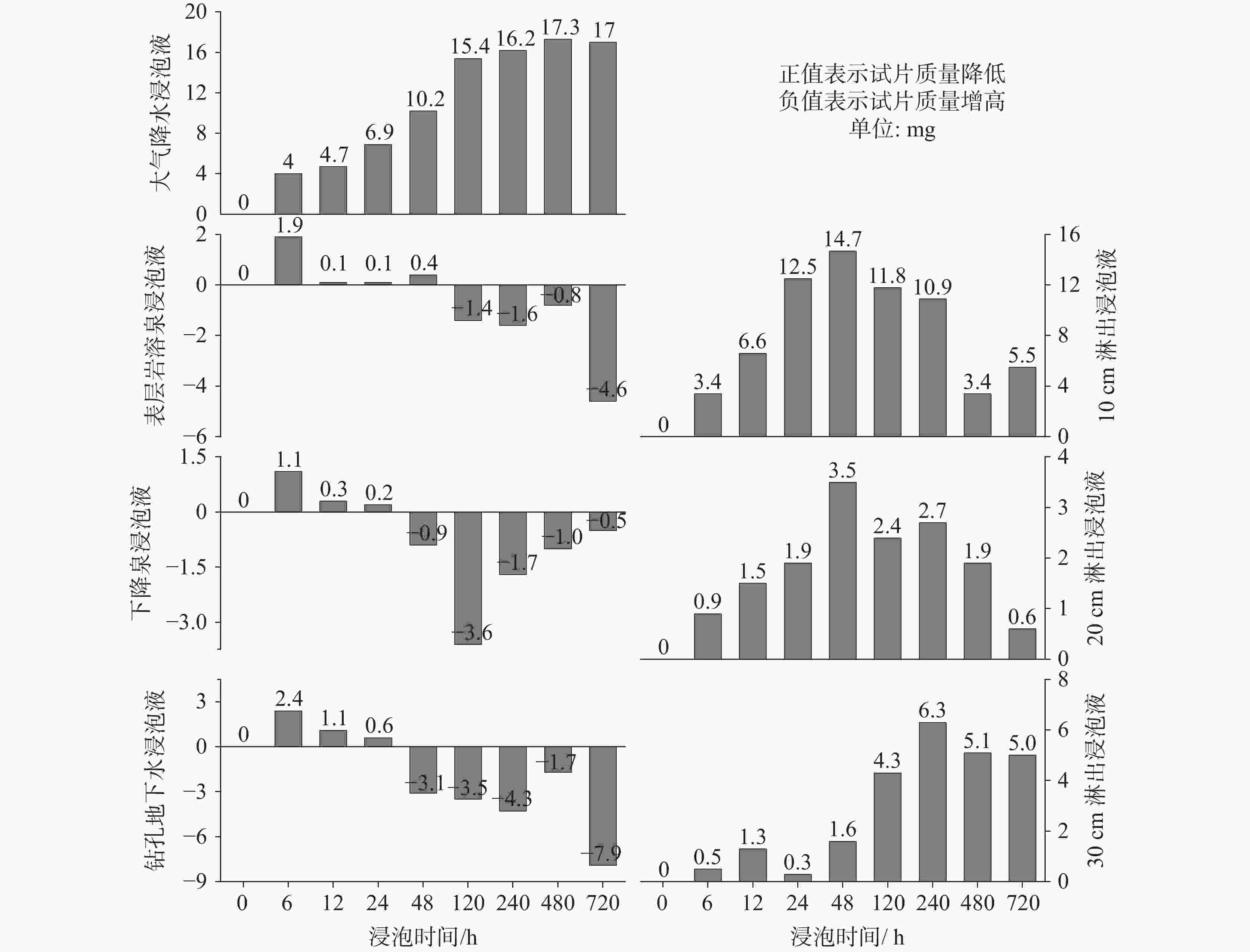
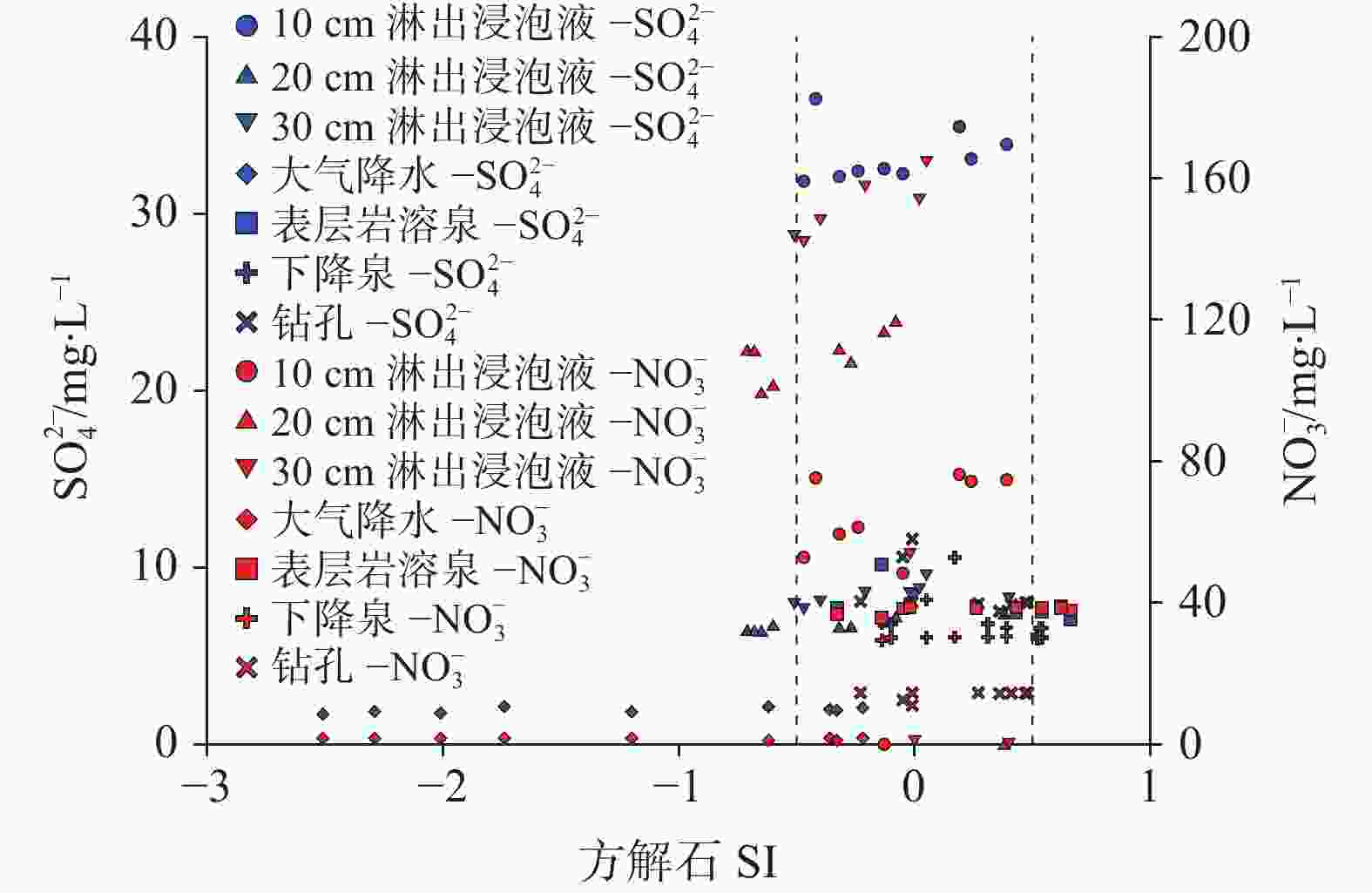
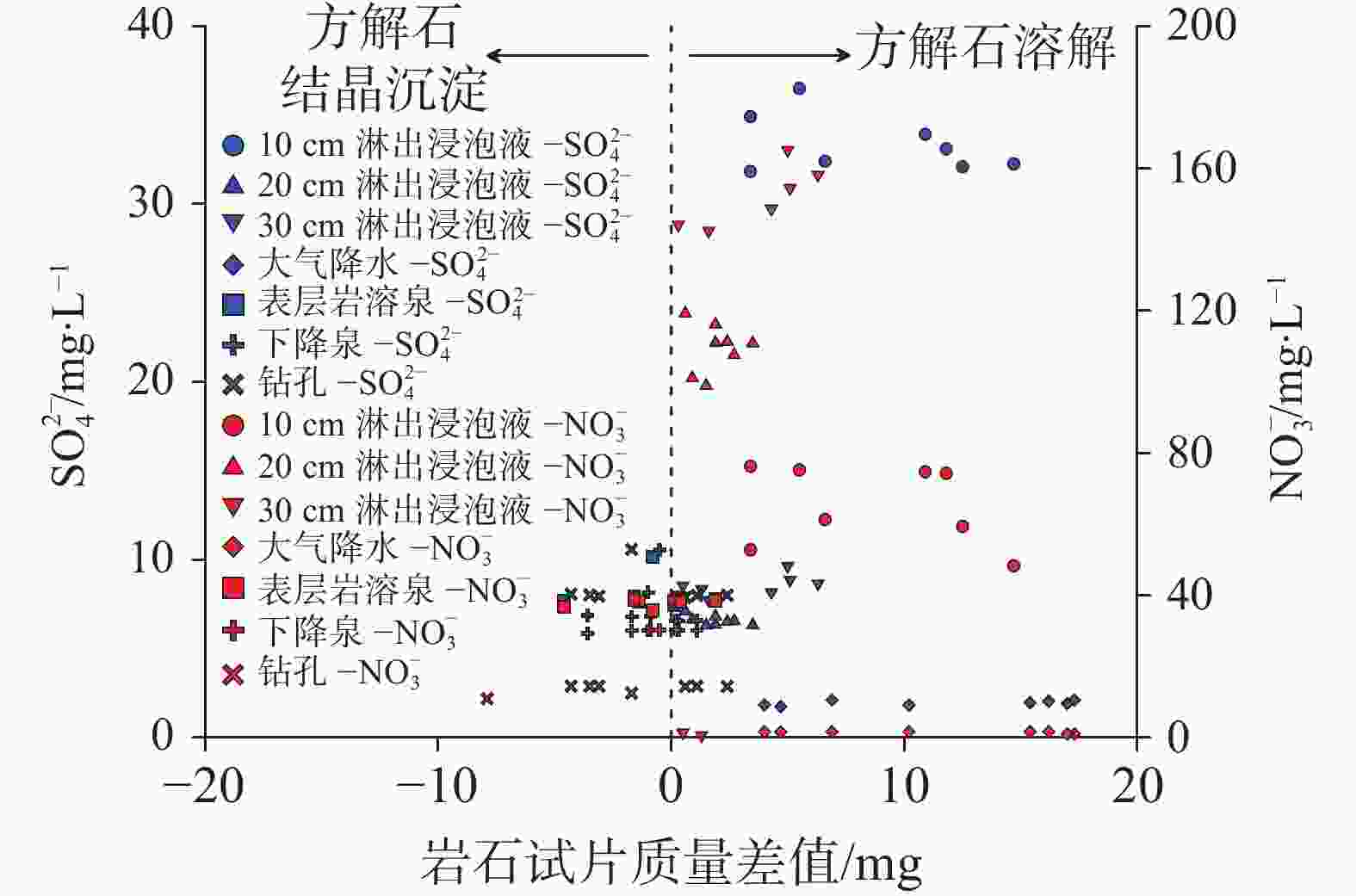
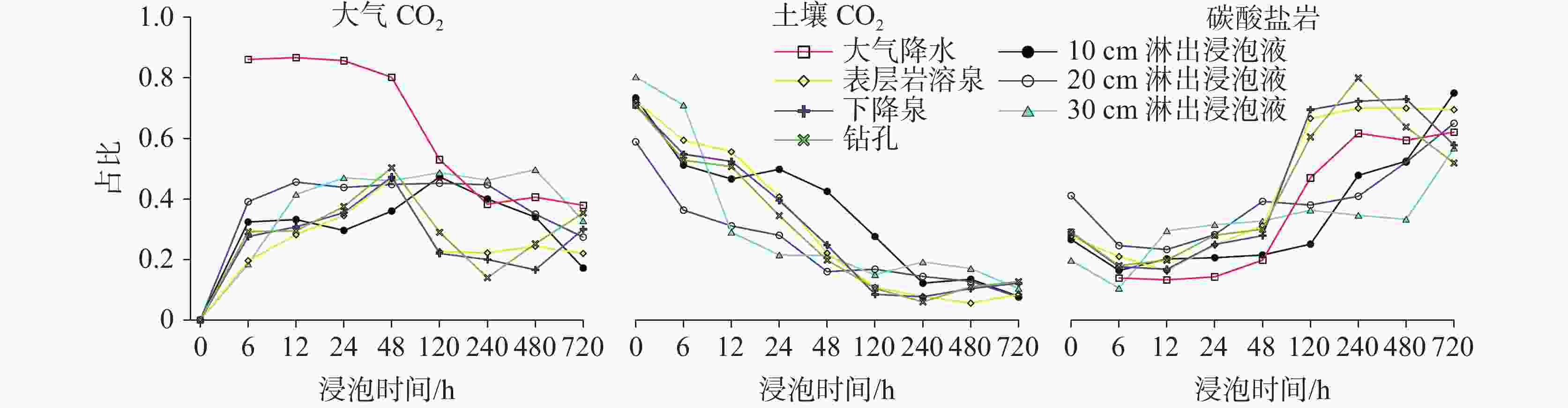
摘要: 碳酸盐岩溶解是一种发生在地球浅表层环境下的特殊地质过程,因其地球化学过程具有低温性、开放性、敏感性和生物参与性等特点,导致岩溶水体溶解无机碳(DIC)浓度及其稳定碳同位素(δ13CDIC)值的多变性。本研究开展大气开放环境中不同水体浸泡碳酸盐岩试片实验,探究大气降水、降水流经岩溶土壤及进入碳酸盐岩含水层再出露地表后对碳酸盐岩溶蚀的影响及δ13CDIC的演变规律。结果表明降水及降水在土壤中渗流再出露地表后,即使无水生光合植物和土壤CO2的持续输入,其对碳酸盐岩仍具有较强的溶蚀作用,而相同条件下岩溶管道/裂隙水出露地表后对碳酸盐岩溶蚀作用微弱或不溶蚀。在无水生光合植物和土壤CO2持续输入的大气开放系统水体中,当${\rm{SO}}_4^{2-}$>29 mg∙L−1或${\rm{NO}}_3^{-}$>50 mg∙L−1时,${\rm{SO}}_4^{2-}$、${\rm{NO}}_3^{-}$产生的盐效应和与阳离子形成的离子对作用可显著提高方解石溶解度,促进方解石溶解。土壤渗流水和岩溶管道/裂隙水出露地表后水中CO2在数小时内即可完成脱气作用,并使水体δ13CDIC值显著增重,增幅可达+10.98‰。
-
关键词:
- 丫吉试验场 /
- 碳酸盐岩溶解 /
- 大气开放环境 /
- 溶解无机碳δ13C同位素 /
- CO2脱气
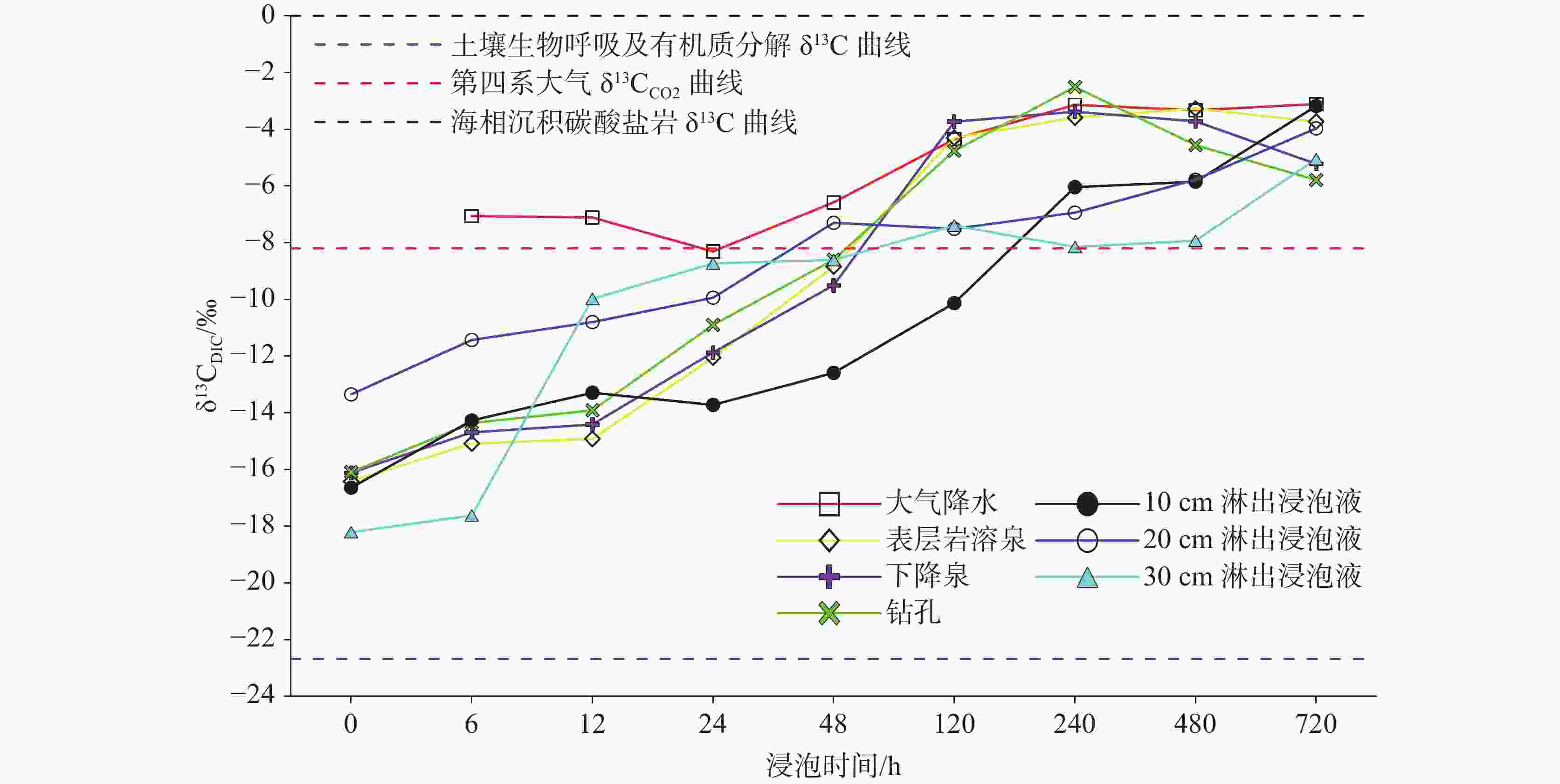
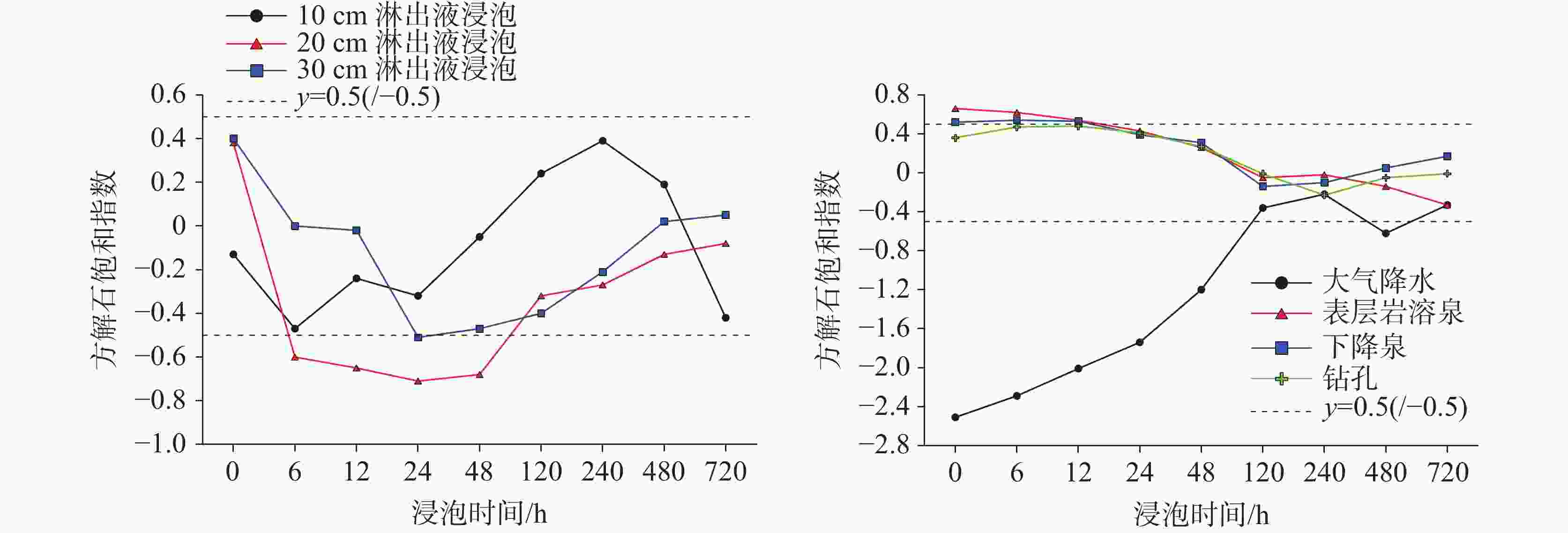
Abstract:Carbonate dissolution is a geological process that occurs in the shallow surface environment of the Earth. Due to its geochemical characteristics—such as low temperature, openness, sensitivity, and biological participation—it leads to variability in the concentration of dissolved inorganic carbon (DIC) and its δ13C (δ13CDIC) values in karst water. In addition, although the global distribution of carbonate rocks (approximately 15.2%) is smaller than that of silicate rocks (approximately 41.8%), the rapid kinetics of carbonate weathering contributes to approximately 68% of DIC in large rivers all over the world. Therefore, exploring the effects of different types of water on the dissolution of carbonate rocks, changes in DIC concentration, and carbon isotope characteristics are of great significance. This study selects the Yaji Karst Experimental Site, a karst area in Southwest China characterized by acid rain, as the research object to conduct experimental investigations in the atmospheric environment. Additionally, this study examines the effects of atmospheric precipitation, soil water, and karst groundwater on this process. The effects of continuous soil CO2 input and aquatic photosynthetic plants are excluded. This study also explores the influence of hydrochemical components in different types of water on the dissolution of carbonate rocks under acid rain conditions. Besides, it examines the control of CO2 degassing and CO2 exchange at the water–gas interface on the evolution of δ13CDIC. The results indicate as follows, (1) Precipitation, along with its infiltration into the soil and subsequent exposure to the surface, still exert a significant dissolution effect on carbonate rocks, even in the absence of a continuous input of soil CO2 and aquatic photosynthetic plants. However, under the same conditions, karst pipelines/fissure water exert weak or no dissolution effects on carbonate rocks after its exposure to the surface. (2) As the soaking time increases, the dissolution amount of carbonate rocks per unit time gradually decreases, and ultimately transforms into calcite crystals, with the highest dissolution rate of approximately 19.7 mg·(cm2·h)−1. Except for the precipitation soaking group, the concentrations of DIC in the other soaking solutions gradually decrease and tend to stabilize. Furthermore, the decrease in DIC in the karst groundwater soaking solution is greater than that in the soil leaching soaking solution. The δ13CDIC values increase progressively with longer soaking time, ranging from an initial value of −18.21‰ to −12.66‰, and reaching values between −5.79‰ and 3.11‰. Except for the precipitation soaking group, the CO2 partial pressure (pCO2) in the other soaking solutions rapidly decreases and tends to stabilize. The stable pCO2 level in each soaking solution is mainly observed: the pCO2 level in soil leaching soaking solution (1,000–3,000 ppmv) is higher than that in karst pipeline/fissure water soaking solution (1,000 ppmv) but is roughly comparable to that in precipitation soaking solution. The level of stable pCO2 in all soaking solutions is higher than that of atmospheric pCO2 (approximately 420 ppmv), with the former being roughly two to eight times higher than the latter. (3) If water is in an open atmospheric system without continuous input of soil CO2 and without aquatic photosynthetic plants, when concentrations of SO$_4^{2-}$ exceed 29 mg∙L−1 or concentrations of NO$_3^{-}$ exceed 50 mg∙L−1, the salt effect and ion pair effect produced by SO$_4^{2-}$ and NO$_3^{-}$ can significantly increase the solubility of calcite and promote its dissolution. If the concentrations of SO$_4^{2-}$ and NO$_3^{-}$ in water are high, the saturation index of calcite, calculated based on Ca2+ and HCO$_3^{-}$ concentrations, may not accurately reflect the dissolution/crystallization state of calcite. Therefore, the simultaneous effects of various ions in water on calcite dissolution/crystallization should be considered, including ion effects, salt effects, and ion pair effects. (4) After soil vadose water or karst pipeline/fissure water is exposed to the surface, CO2 in the water can complete the degassing process within a few hours. In the absence of a continuous input of soil CO2 and aquatic photosynthetic plants, pCO2 quickly reaches an equilibrium state and ceases to be the major factor controlling the dissolution/crystallization of calcite. The CO2 degassing effect significantly increases the δ13CDIC value, with an increase of up to +10.98 ‰. This results in a substantial discrepancy between the actual and theoretical contributions of soil or atmospheric CO2 to DIC. -
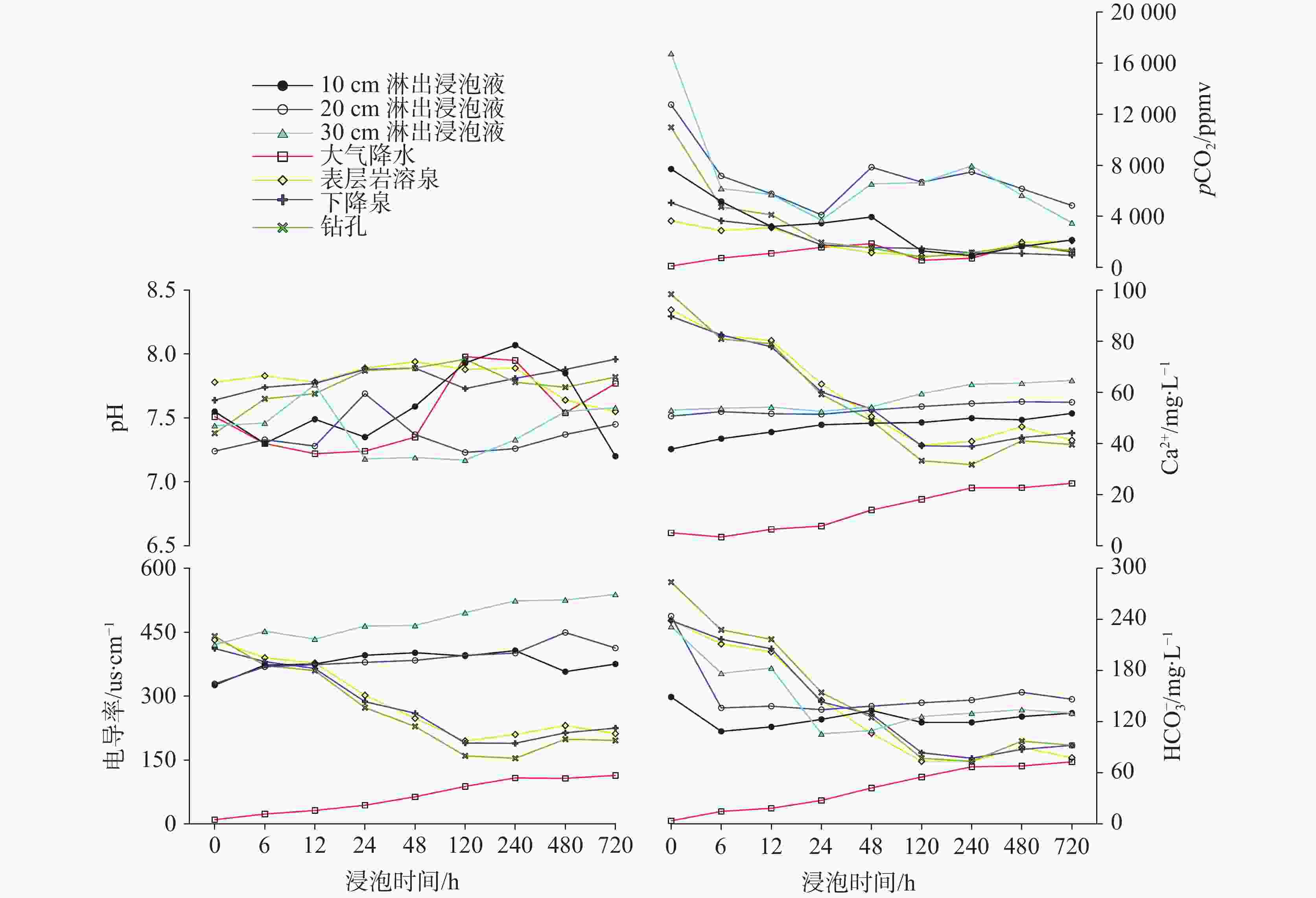
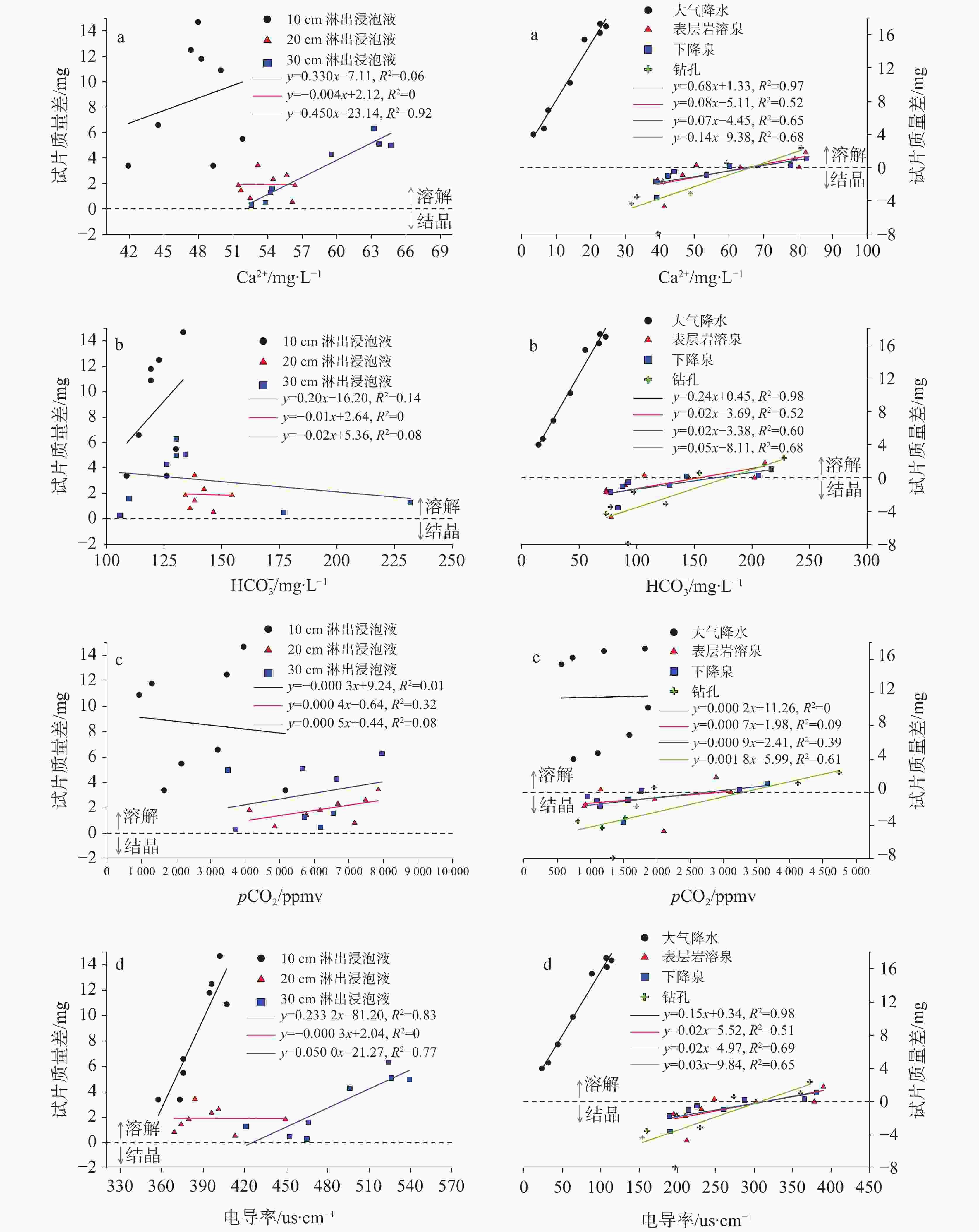
图 7 不同类型浸泡液中主要水化学指标与试片质量差关系(除初始浸泡液;a-Ca2+与试片质量差;b-${\rm{HCO}}_3^{-}$与试片质量差;c- pCO2与试片质量差;d-电导率与试片质量差)
Figure 7. Relationship between major hydrochemical indexes of the soaking solutions and quality difference of rock tablets. (Except for the initial soaking solution; a: Ca2+ vs. quality difference of rock tablets; b: ${\rm{HCO}}_3^{-}$ vs. quality difference of rock tablets; c: pCO2 vs. quality difference of rock tablets; d: conductivity vs. quality difference of rock tablets)
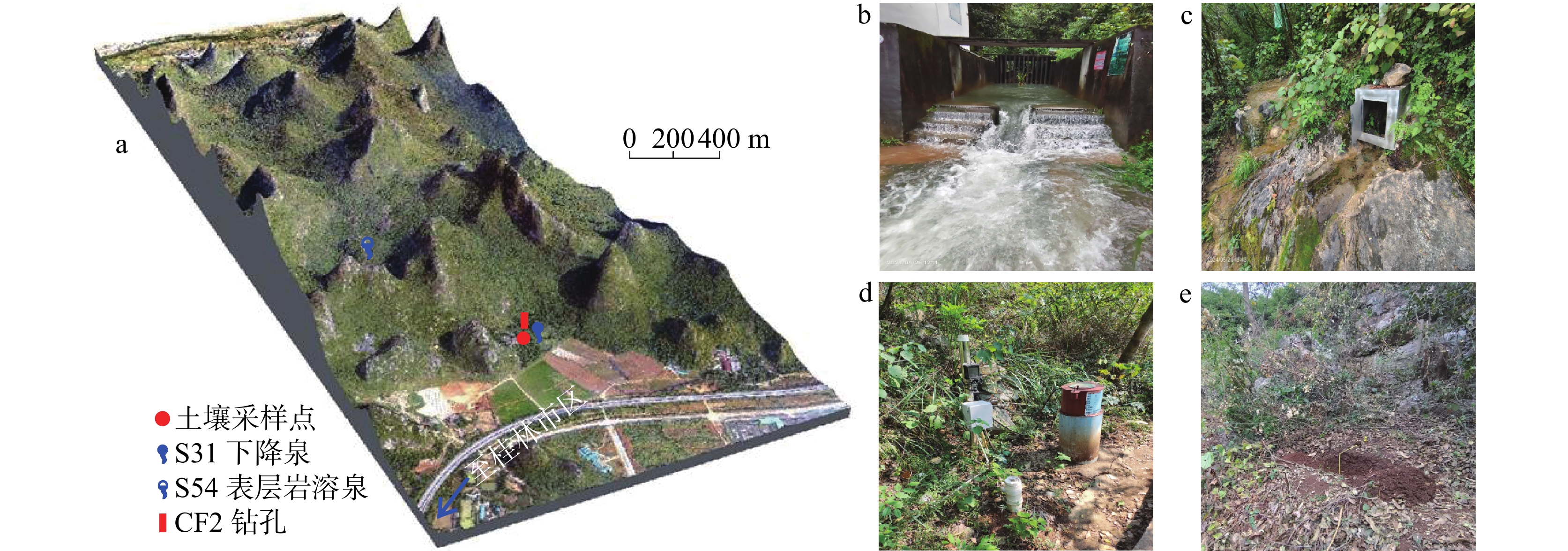
表 1 桂林丫吉试验场坡面土壤主要理化指标含量
Table 1. Contents of major physicochemical indicators in the slope soil of Yaji Experimental Site
深度/cm pH 容重/
g∙cm−3含水率/% 水溶性盐总量/
mg∙kg−1阳离子交换量/
cmol∙kg−1碳酸钙/
g∙kg−1有机质/
g∙kg−1δ13C有机碳/‰ δ13C无机碳/‰ 0~10 6.66 1.18 29.8 100 30.5 5.97 72.6 −21.36 −13.45 10~20 6.34 1.02 31.2 120 30.4 9.36 55.9 −19.24 −12.88 20~30 6.42 0.93 32.7 190 30.4 11.4 45.2 −17.03 −16.22 表 2 岩石试片尺寸及主要化学成分
Table 2. Sizes and major chemical components of rock tablets
试片岩性 采样点 形状 直径/mm 高度/mm 表面积/cm2 CaO MgO CO2 酸不溶物 质量分数/% 灰岩 桂林市七星岩 圆饼状 40 3 28.9 55.69 0.42 42.97 0.67 表 3 浸泡液水化学指标检测结果统计
Table 3. Statistics of testing results of water chemical indexes in soaking solutions
浸泡液
类型pH Ca2+ Mg2+ K+ Na+ ${\rm{HCO}}_3^{-}$ ${\rm{SO}}_4^{2-}$ ${\rm{NO}}_3^{-}$ Cl− 电导率 pCO2 δ13CDIC mg·L−1 us·cm−1 ppmv ‰ L10
(n=9)最大值 8.07 51.80 17.48 6.02 2.46 149.00 36.50 76.30 6.54 407.00 7709 −3.18 最小值 7.20 37.81 15.10 4.69 1.38 108.69 31.84 / 4.58 326.00 931 −16.64 平均值 7.59 46.52 16.06 5.09 1.74 124.68 33.29 − 5.01 378.56 3281 −10.63 标准差 0.30 4.39 0.87 0.50 0.38 11.89 1.55 − 0.61 25.43 2152 4.60 变异系数 3.95 9.43 5.40 9.76 21.74 9.54 4.67 − 12.19 6.72 66 −43.28 L20
(n=9)最大值 7.74 56.36 18.55 3.58 2.97 243.96 7.38 119.72 7.86 449.50 12749 −3.97 最小值 6.88 50.72 17.45 3.11 2.56 134.18 6.41 / 7.23 329.00 4121 −13.35 平均值 7.19 53.56 17.84 3.34 2.78 153.27 6.76 − 7.51 388.33 6982 −8.56 标准差 0.30 2.16 0.39 0.13 0.13 34.58 0.35 − 0.19 33.10 2481 3.00 变异系数 4.13 4.02 2.16 3.99 4.80 22.56 5.13 − 2.55 8.52 36 −35.11 L30
(n=9)最大值 7.76 64.70 23.34 1.40 2.72 231.78 9.56 164.67 9.64 539.00 16749 −5.05 最小值 7.17 52.57 21.46 0.94 1.97 105.73 7.65 0.19 8.60 421.00 3499 −18.21 平均值 7.41 57.68 22.28 1.07 2.17 147.52 8.43 107.26 8.89 480.39 6963 −10.19 标准差 0.21 5.05 0.57 0.14 0.23 41.28 0.55 68.79 0.31 42.66 3928 4.58 变异系数 2.78 8.75 2.57 13.35 10.56 27.98 6.55 64.13 3.54 8.88 56 −44.94 CP
(n=9)最大值 7.98 24.43 0.14 0.13 0.47 72.90 2.13 1.76 0.83 114.00 1919 −3.11 最小值 7.22 3.42 0.04 0.09 / 3.67 1.70 1.11 0.46 9.89 114 −12.66 平均值 7.54 13.84 0.08 0.11 − 41.07 1.93 1.58 0.73 65.49 1092 −6.18 标准差 0.30 8.41 0.04 0.01 − 26.00 0.15 0.26 0.12 40.16 615 3.13 变异系数 3.93 60.77 46.95 12.90 − 63.29 8.01 16.51 16.70 61.33 56 −50.65 CS54
(n=9)最大值 7.94 92.31 0.61 0.15 0.82 238.81 10.16 38.75 3.03 432.00 3648 −3.27 最小值 7.55 39.31 0.41 0.03 0.04 73.48 7.04 35.87 2.65 195.00 904 −16.42 平均值 7.80 59.62 0.46 0.10 0.45 135.38 7.91 37.98 2.87 288.67 2050 −9.13 标准差 0.13 20.54 0.06 0.03 0.27 66.03 0.89 0.98 0.15 89.99 992 5.57 变异系数 1.65 34.44 13.36 35.13 60.51 48.77 11.24 2.59 5.41 31.18 48 −60.99 CS31
(n=9)最大值 7.96 89.76 0.77 0.33 0.92 238.81 10.57 30.45 2.72 412.00 5070 −3.38 最小值 7.64 38.89 0.61 0.05 / 77.15 6.19 29.36 2.29 189.40 959 −16.11 平均值 7.81 58.69 0.66 0.17 − 141.53 7.25 30.10 2.57 280.38 2221 −9.18 标准差 0.10 19.95 0.06 0.09 − 63.50 1.36 0.33 0.14 85.87 1432 5.27 变异系数 1.28 34.00 9.06 54.99 − 44.87 18.78 1.08 5.42 30.63 64 −57.37 CCF2
(n=9)最大值 7.96 98.43 0.65 0.38 1.07 283.82 11.60 14.54 2.94 441.00 10965 −2.51 最小值 7.38 31.74 0.40 0.21 0.14 73.48 7.52 11.01 2.57 154.10 809 −16.09 平均值 7.75 56.90 0.46 0.30 0.54 149.75 8.63 13.88 2.78 264.88 3146 −9.05 标准差 0.17 23.99 0.08 0.05 0.30 76.11 1.43 1.25 0.13 103.25 3230 4.96 变异系数 2.21 42.17 17.92 18.21 55.19 50.82 16.55 9.02 4.84 38.98 103 −54.81 -
[1] Goldscheider N, Chen Z, Auler A S, Bakalowicz M, Broda S, Drew D, Hartmann J, Jiang G, Moosdorf N, Stevanovic Z, Veni G. Global distribution of carbonate rocks and karst water resources[J]. Hydrogeology Journal, 2020, 28: 1661-1677. [2] Zhang S, Bai X, Zhao C, Tan Q, Luo G, Wang J, Li Q, Wu L, Chen F, Li C, Deng Y, Yang Y, Xi H. Global CO2 consumption by silicate rock chemical weathering: Its past and future[J]. Earth's Future, 2021, 9: e2020EF001938. [3] Dreybrodt W, Buhmann D, Michaelis J, Usdowski E. Geochemically controlled calcite precipitation by CO2-outgassing: Field measurements of precipitation rates in comparison to theoretical predictions[J]. Carsologica Sinica, 1992, S1: 9-24. [4] 刘再华, Dreybrodt W. 方解石沉积速率控制的物理化学机制及其古环境重建意义[J]. 中国岩溶, 2002, 21(4):252-257. doi: 10.3969/j.issn.1001-4810.2002.04.004LIU Zaihua, Dreybrodt W. Physicochemical mechanisms of rate-determining of calcite deposition and their implications for paleo-environmental reconstruction[J]. Carsologica Sinica, 2002, 21(4): 252-257. doi: 10.3969/j.issn.1001-4810.2002.04.004 [5] 闫志为. 硫酸根离子对方解石和白云石溶解度的影响[J]. 中国岩溶, 2008, 27(1):24-31. doi: 10.3969/j.issn.1001-4810.2008.01.005YAN Zhiwei. Influences of ${\rm{SO}}_4^{2-}$ on the solubility of calcite and dolomite[J]. Carsologica Sinica, 2008, 27(1): 24-31. doi: 10.3969/j.issn.1001-4810.2008.01.005 [6] Jiang Y J, Hu Y J, Schirmer M. Biogeochemical controls on daily cycling of hydrochemistry and δ13C of dissolved inorganic carbon in a karst spring-fed pool[J]. Journal of Hydrology, 2013, 478: 157-168. doi: 10.1016/j.jhydrol.2012.12.001 [7] Pu J B, Li J H, Khadka M B, Martin J B, Zhang T, Yu S, Yuan D. In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream[J]. Science of the Total Environment, 2017, 579: 1343-1355. doi: 10.1016/j.scitotenv.2016.11.132 [8] Zhang T, Li J H, Pu J B, Martin J B, Khadka M B, Wu F, Li L, Jiang F, Huang S, Yuan D. River sequesters atmospheric carbon and limits the CO2 degassing in karst area, southwest China[J]. Science of the Total Environment, 2017, 609: 92-101. doi: 10.1016/j.scitotenv.2017.07.143 [9] Omelon C R, Pollard W H, Andersen D T. A geochemical evaluation of perennial spring activity and associated mineral precipitates at Expedition Fjord, Axel Heiberg Island, Canadian High Arctic[J]. Applied Geochemistry, 2006, 21: 1-15. doi: 10.1016/j.apgeochem.2005.08.004 [10] 周小萍, 蓝家程, 张笑微, 徐尚全. 岩溶溪流的脱气作用及碳酸钙沉积: 以重庆市南川区柏树湾泉溪流为例[J]. 沉积学报, 2013, 31(6):1014-1021.ZHOU Xiaoping, LAN Jiacheng, ZHANG Xiaowei, XU Shangquan. CO2 outgassing and precipitation of calcium carbonate in a karst stream: A case study of Baishuwan spring in Nanchuan, Chongqing[J]. Acta Sedimentologica Sinica, 2013, 31(6): 1014-1021. [11] 章程, 肖琼. 桂林漓江水体溶解无机碳迁移与水生光合碳固定研究[J]. 中国岩溶, 2021, 40(4):555-564.ZHANG Cheng, XIAO Qiong. Study on dissolved inorganic carbon migration and aquatic photosynthesis sequestration in Lijiang River, Guilin[J]. Carsologica Sinica, 2021, 40(4): 555-564. [12] 袁道先, 刘再华, 林玉石, 等. 中国岩溶动力系统[M]. 北京: 地质出版社, 2002:1−275.YUAN Daoxian, LIU Zaihua, LIN Yushi, et al. Karst dynamic systems of China[M]. Beijing: Geology Press, 2002:1−275. [13] Li W, Yu L J, Yuan D X, Xu H B, Yang Y. Bacteria biomass and carbonic anhydrase activity in some karst areas of southwest China[J]. Journal of Asian Earth Sciences, 2004, 24: 145-152. doi: 10.1016/j.jseaes.2003.10.008 [14] Zhang C, Yuan D X, Cao J H. Analysis of the environmental sensitivities of a typical dynamic epikarst system at the Nongla monitoring site, Guangxi, China[J]. Environmental Geology, 2005, 47: 615-619. doi: 10.1007/s00254-004-1186-x [15] 章程, 袁道先. IGCP448: 岩溶生态系统全球对比研究进展[J]. 中国岩溶, 2005, 24(1):83-88.ZHANG Cheng, YUAN Daoxian. IGCP448: Progresses of world correlation of karst ecosystem[J]. Carsologica Sinica, 2005, 24(1): 83-88. [16] 黄奇波, 吴华英, 程瑞瑞, 李腾芳, 罗飞, 赵光帅, 李小盼. 桂林岩溶区石灰土壤对酸雨缓冲作用的观测及其对岩溶碳汇的指示意义[J]. 地球学报, 2022, 43(4): 461−471.HUANG Qibo, WU Huaying, CHENG Ruirui, LI Tengfang, LUO Fei, ZHAO Guangshuai, LI Xiaopan. Buffering effect of lime soil on acid rain and its influence on the evaluation of the karst carbon sink effect. Acta Geoscientica Sinica, 2022, 43(4): 461−471. [17] 赵光帅, 黄奇波, 朱义年, 李腾芳, 普政功. 石灰土对硫酸型酸雨缓冲过程模拟及碳汇效应研究[J]. 中国岩溶, 2022, 41(5):796-807. doi: 10.11932/karst20220504ZHAO Guangshuai, HUANG Qibo, ZHU Yinian, LI Tengfang, PU Zhenggong. Simulation of buffering process and carbon sink effect of lime soil on sulfuric acid rain[J]. Carsologica Sinica, 2022, 41(5): 796-807. doi: 10.11932/karst20220504 [18] Zhao G, Huang Q, Zhu Y, Xu Y, Pu Z. Simulation of the buffering process of karst soil on sulfuric acid rain and the characteristic of δ13CDIC and the carbon sink flux in Guilin City, southwest China[J]. Environmental Earth Sciences, 2023, 82: 296. doi: 10.1007/s12665-023-10948-6 [19] Cuntz M. Carbon cycle: a dent in carbon's gold standard[J]. Nature, 2011, 477: 547-548. doi: 10.1038/477547a [20] Yang H, Zhou L, Huang L, Cao J, Groves C. A comparative study of soil carbon transfer between forest soils in subtropical karst and clasolite areas and the karst carbon sink effect in Guilin, Guangxi, China[J]. Environmental Earth Sciences, 2015, 74: 921-928. doi: 10.1007/s12665-014-3903-4 [21] 闫志为, 刘辉利, 张志卫. 温度及CO2对方解石、白云石溶解度影响特征分析[J]. 中国岩溶, 2009, 28(1):7-10.YAN Zhiwei, LIU Huili, ZHANG Zhiwei. Influences of temperature and PCO2 on the solubility of calcite and dolomite[J]. Carsologica Sinica, 2009, 28(1): 7-10. [22] Shin W J, Chung G S, Lee D, Lee K S. Dissolved inorganic carbon export from carbonate and silicate catchments estimated from carbonate chemistry and δ13CDIC[J]. Hydrology and Earth System Sciences, 2011, 15: 2551-2560. doi: 10.5194/hess-15-2551-2011 [23] Palmer S M, Hope D, Billett M F, Dawson J J C, Bryant C L. Sources of organic and inorganic carbon in a headwater stream: Evidence from carbon isotope studies[J]. Biogeochemistry, 2001, 52: 321-338. doi: 10.1023/A:1006447706565 [24] Doctor D H, Kendall C, Sebestyen S D, Shanley J B, Ohte N, Boyer E W. Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream[J]. Hydrological Processes, 2008, 22: 2410-2423. doi: 10.1002/hyp.6833 -




 下载:
下载: