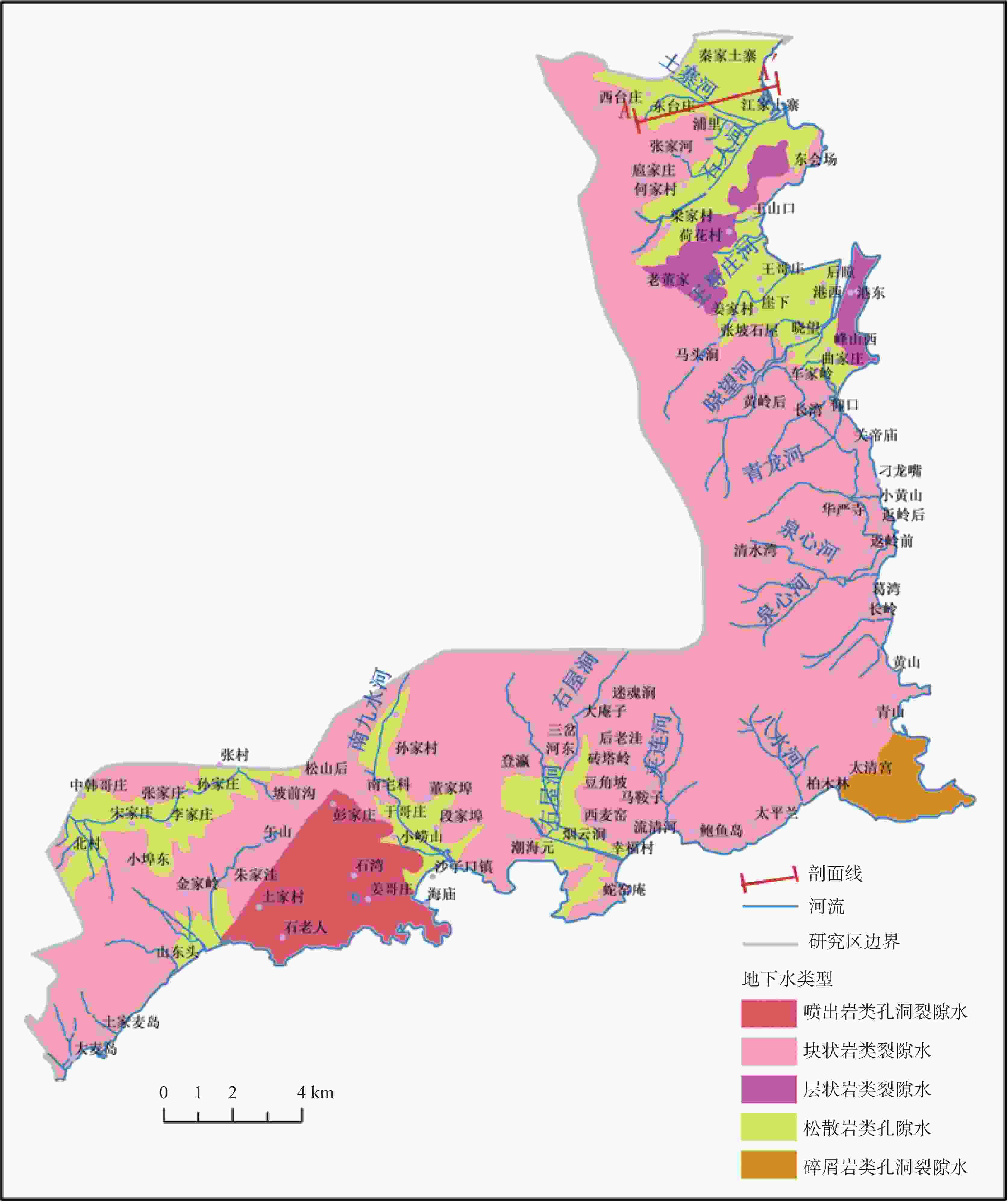
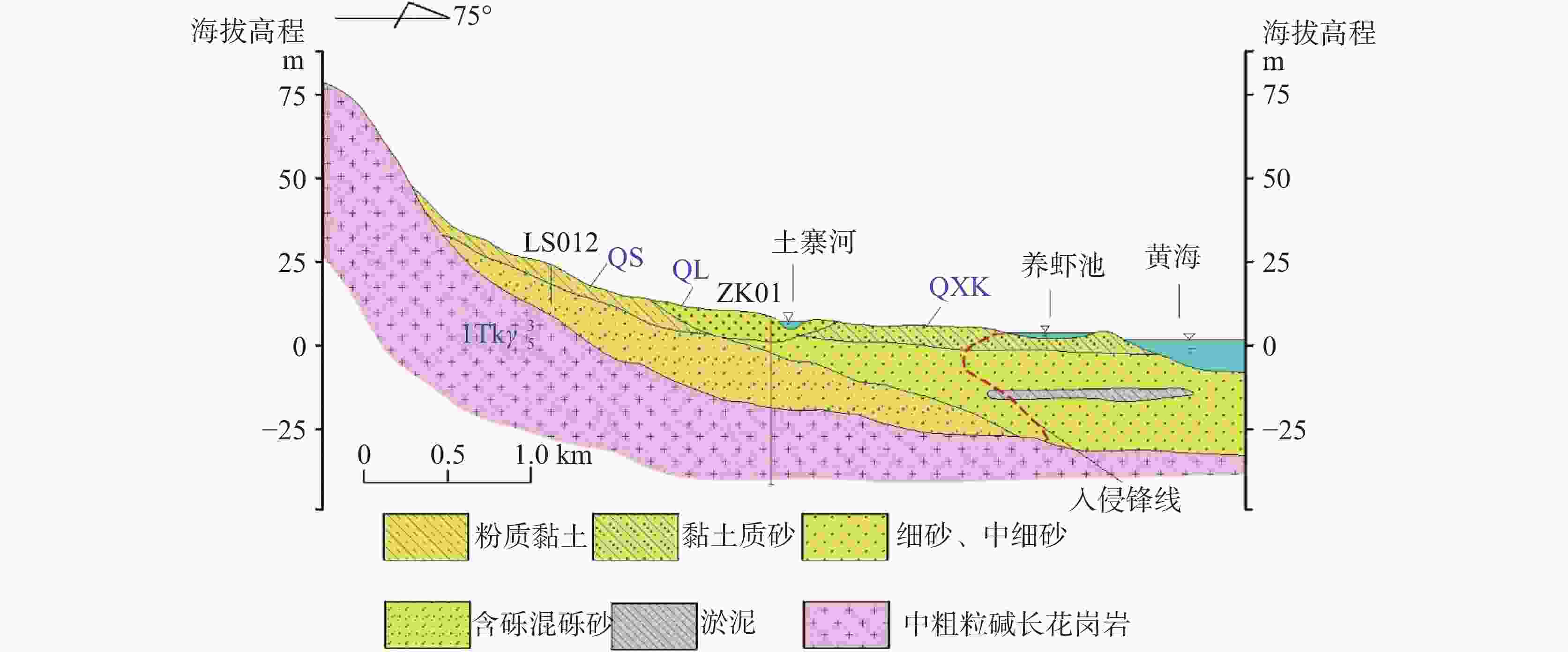
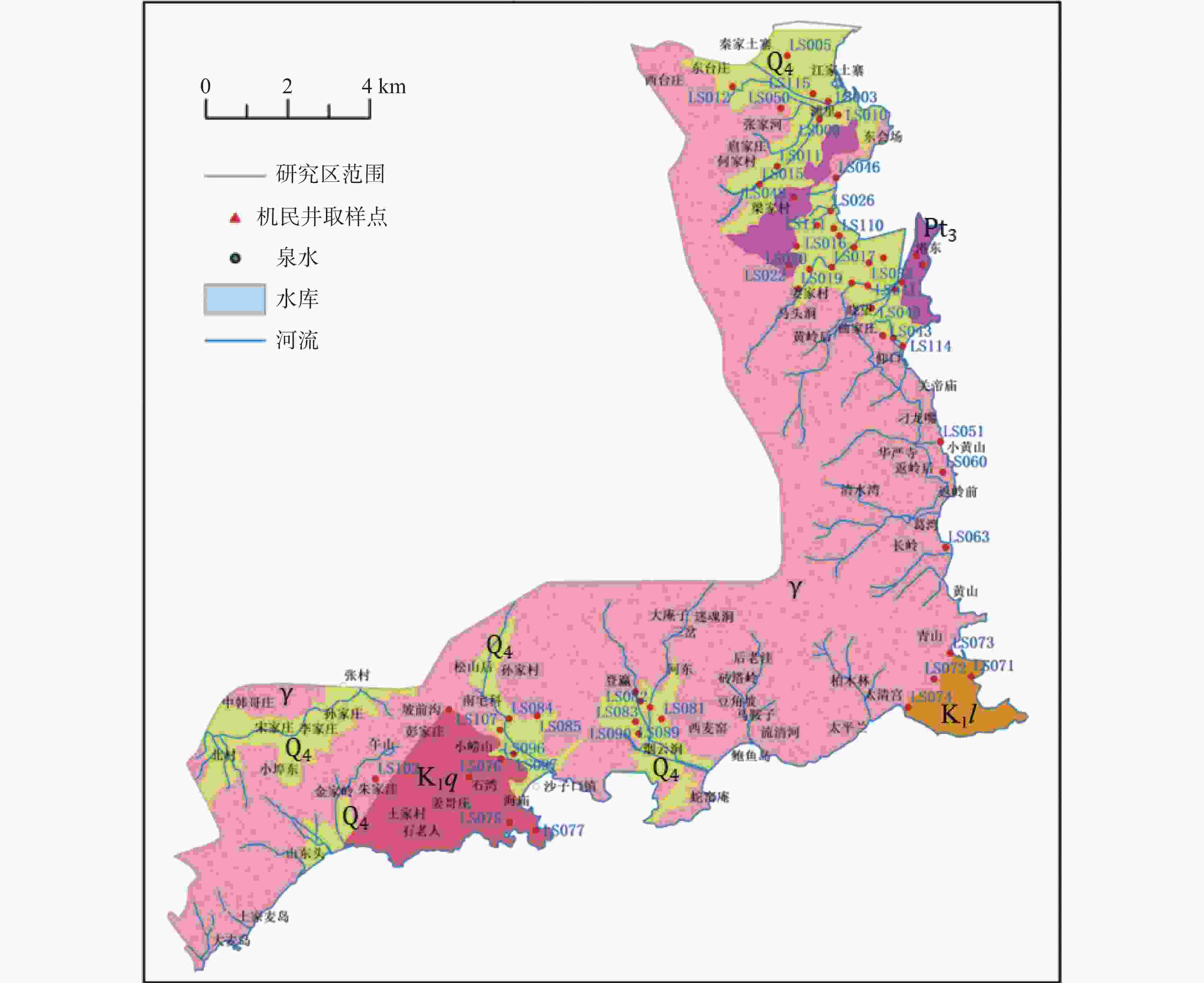
Characterization of seawater intrusion based on multivariate statistical analysis and water chemistry characteristics: A case study of Laoshan district, Qingdao City
-
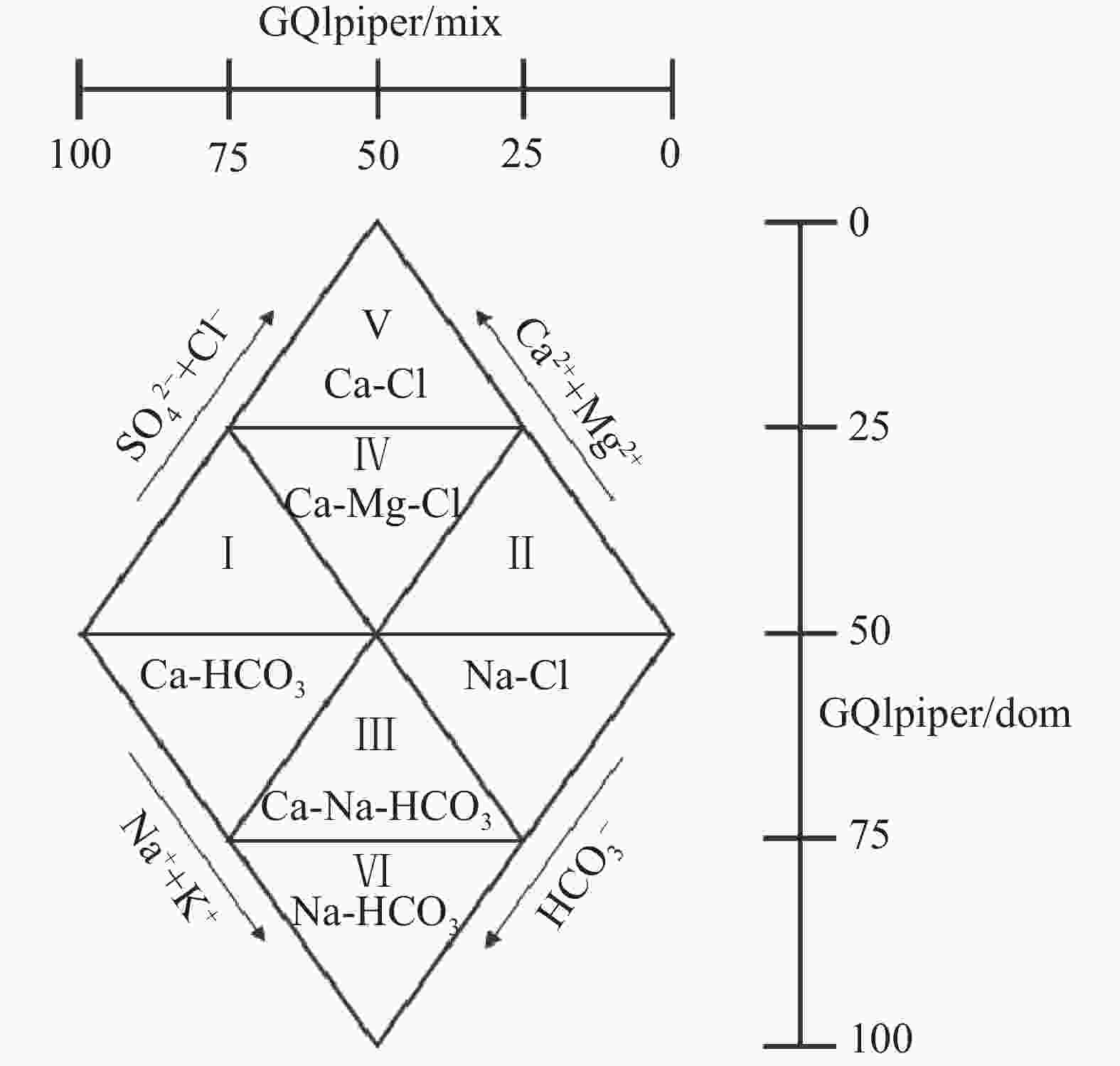
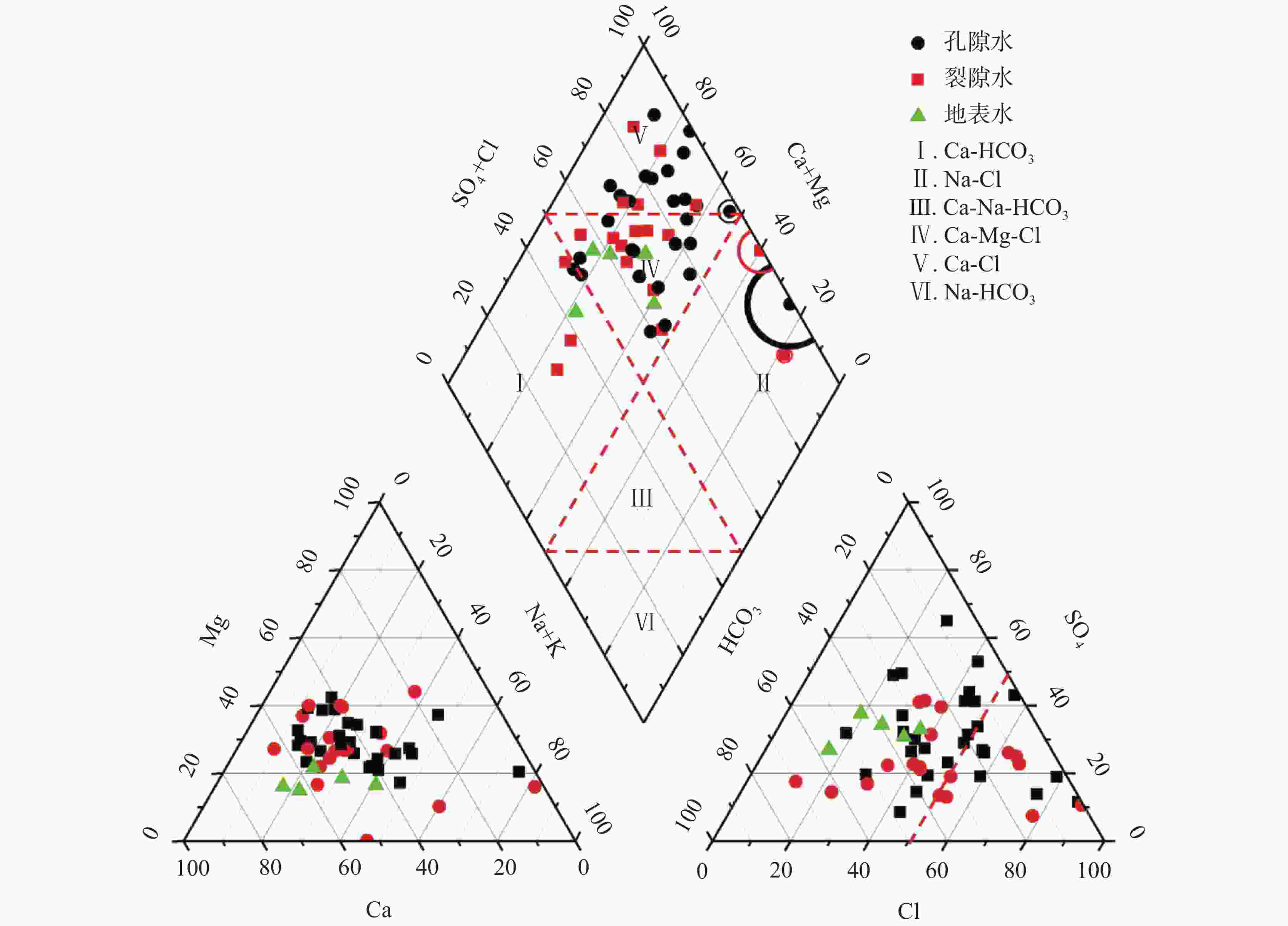
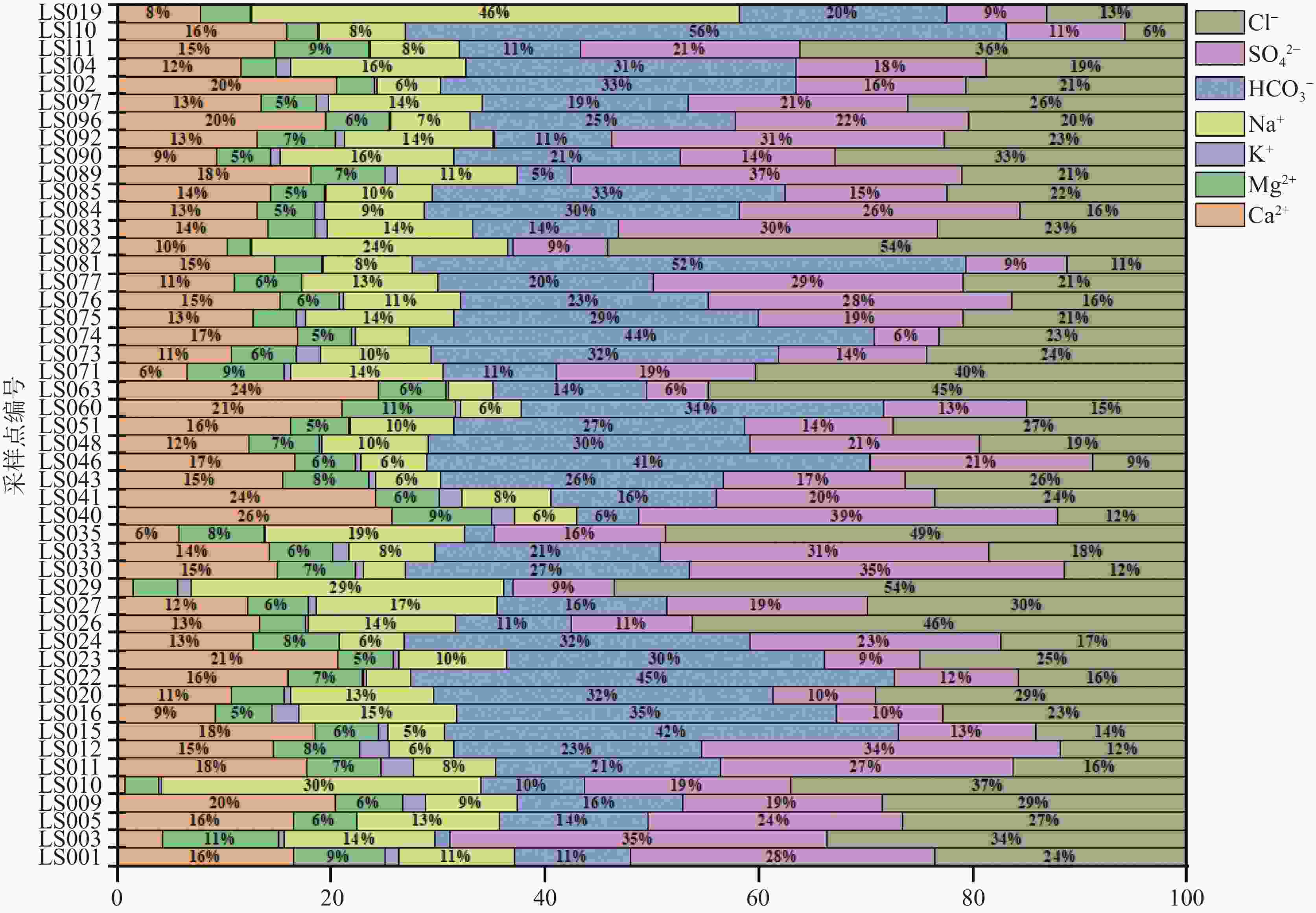
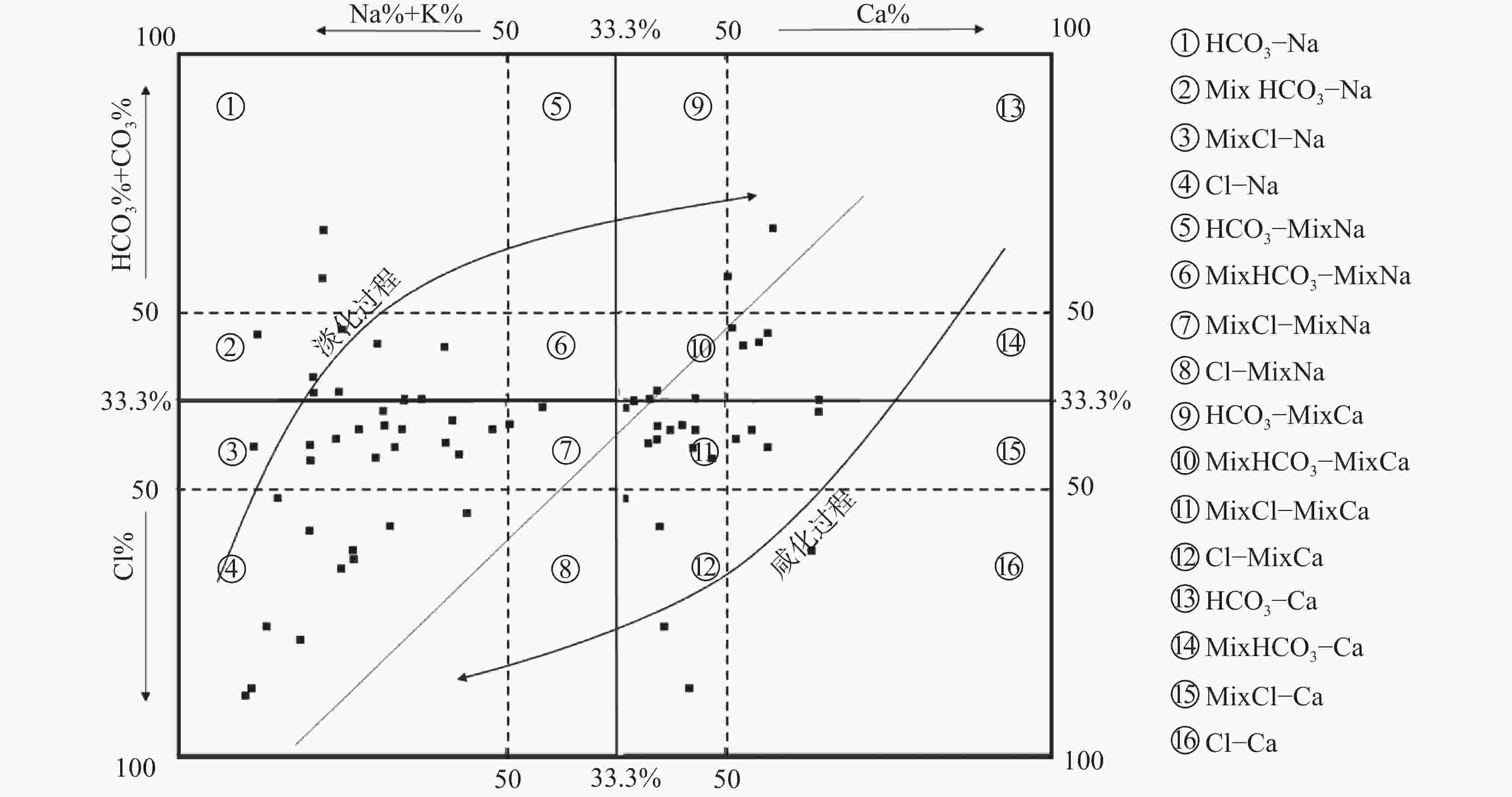
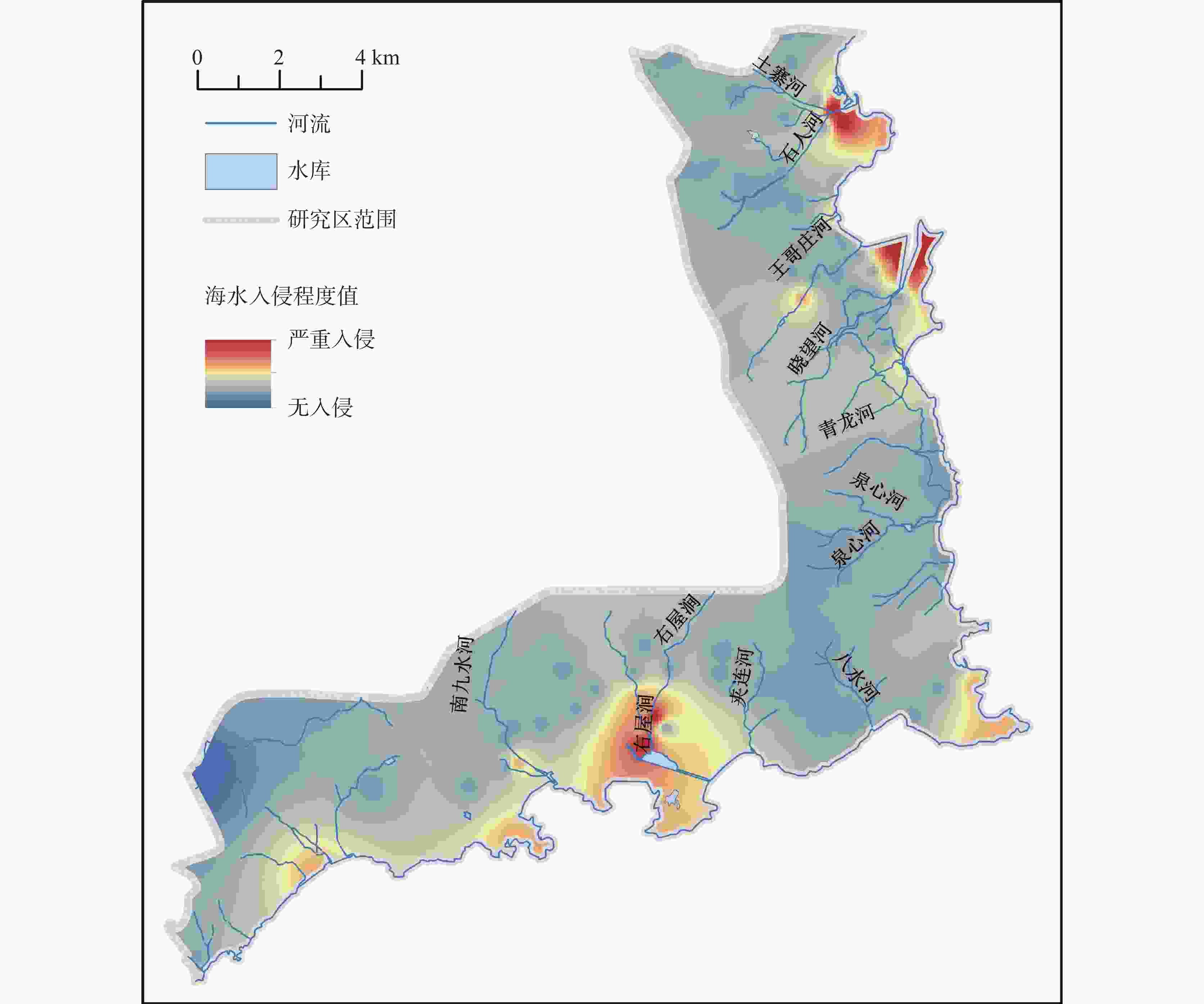
摘要: 沿海地区地下水环境问题日益突出,进行地下水水化学特征及演化规律的研究,能够更有效地开展地下水环境的监测和保护。以青岛市崂山区地下水为研究对象,综合运用统计分析、主成分分析、Piper图解法、HFE-D图解法、Chadha’s矩形图法等方法,对研究区海水入侵特征与地下水化学特征演化进行分析,探究地下水水化学特征及演化规律,并进一步评价了海水入侵现状。结果表明,研究区地下水以Na+、Ca2+、Cl−、${\rm{SO}}_4^{2-}$为主要优势离子,地下水化学类型多为Cl·SO4-Na型和SO4·Cl-Ca·Mg型。地下水中Cl−浓度变化幅度较大,且其均值超出了有无海水入侵的分界值(250 mg·L−1),地下水可能发生一定程度的海水入侵;青岛市崂山区地下水呈中性至弱碱性(pH均值=7.0~8.0),是沿海地区长期的水文地球化学过程的影响;地下水化学变化主要受自然因素(岩石与水的相互作用)或人为因素(农业和家庭活动)的控制;采用反距离加权(IDW)方法,结合地理信息系统(GIS),进行海水入侵位置的空间映射,研究结果表明崂山区海水入侵主要分布于江家土寨东−浦里社区北入侵段,王哥庄−港西−港东入侵段、仰口湾入侵段、登瀛村−栲栳岛入侵段。Abstract:
Groundwater is an important source of freshwater in coastal areas. With the rapid development of industrialization and urbanization, the water demand for production and living in coastal areas has been rising year by year. Therefore, the increasing exploitation of groundwater has triggered seawater intrusion and increasingly prominent environmental problems of groundwater in many places. Researching the hydro-chemical characteristics and evolutionary patterns of groundwater enables effective monitoring and protection of the groundwater environment. Taking the groundwater in Laoshan district, Qingdao City as the research object, we mainly focused on the issues of groundwater chemical characteristics, groundwater chemical processes, the degree of seawater intrusion and its impact on groundwater. In addition, under the theoretical guidance of hydrogeology, we analyzed the characteristics of seawater intrusion and evolution of groundwater hydrochemistry in the study area by means of data collection, theoretical analysis, field investigation and sample collection and testing. The research findings can provide a scientific basis for the rational development and utilization of groundwater in the area. The results show that the groundwater in the study area has Na+, Ca2+, Cl−, and ${\rm{SO}}_4^{2-}$ as the main dominant ions, and most of the groundwater chemistry types are Cl·SO4-Na and SO4·Cl-Ca·Mg types. The Cl− concentration in the groundwater varied considerably and its mean value exceeded the cut-off value for the presence or absence of seawater intrusion (250 mg·L−1), indicating that some degree of seawater intrusion may have occurred in groundwater. Groundwater in the Laoshan district of Qingdao City is neutral to weakly alkaline (mean pH=7.0–8.0), which is an effect of long-term hydrogeochemical processes in the coastal area. The results obtained by the PCA model show that changes in groundwater chemistry are mainly controlled by natural factors (rock-water interaction) or anthropogenic factors (agricultural and domestic activities). The five chemical characteristics of Cl−, mineralization, ${\rm{SO}}_4^{2-}$, γCl−/γHCO$_3^- $ and SAR were selected as evaluation factors. Based on the inverse distance weighting (IDW) method and geographic information systems (GIS), we achieved the spatial mapping of seawater intrusion locations, showing that the seawater intrusion, in Laoshan district was mainly distributed in the intrusion sections such as the east of Jiangjia Tuzhai—the north of Puli community, the area of Wanggezhuang-Gangxi-Gangdong, Yangkou bay and the area of Danying village-Quanzhou island. The study results are of great significance for the use of groundwater resources and the prevention and control of seawater intrusion in Laoshan district. In addition, the research ideas and methods provide a reference for the study of groundwater genesis in other coastal areas in the world. -
表 1 地下水水化学参数统计特征值(单位:mg·L−1,pH除外)
Table 1. Statistics of hydrochemical parameters of groundwater (unit: mg·L−1, except for pH)
分区 项目 pH TDS TH Ca2+ Mg2+ K+ Na+ ${\rm{HCO}}_3^{-}$ ${\rm{SO}}_4^{2-}$ Cl− ${\rm{NO}}_3^{-}$ 基岩裂
隙水Mean 7.34 998.81 380.60 94.23 34.71 1.78 199.04 104.33 135.62 385.94 35.94 SD 0.27 2032.16 670.59 200.68 46.61 2.49 512.01 82.42 218.81 1103.45 32.57 Cv 0.04 2.03 1.76 2.13 1.34 1.40 2.57 0.79 1.61 2.86 0.91 Min 6.90 67.21 46.12 12.93 3.36 0.13 2.17 7.65 3.05 21.53 0 Max 8.20 8 877.64 3 064.85 910.97 191.90 8.82 2 125.00 306.08 778.46 4 812.71 101.35 第四系
孔隙水Mean 7.20 1 435.83 554.26 88.35 81.04 12.92 277.95 108.77 235.23 560.83 69.69 SD 0.38 3 211.69 802.72 63.12 163.27 36.36 927.56 75.61 403.37 1 722.63 67.98 Cv 0.05 2.24 1.45 0.71 2.01 2.81 3.34 0.70 1.71 3.07 0.98 Min 6.50 74.83 39.20 8.31 4.48 0.17 6.67 15.30 10.68 13.13 0.36 Max 8.50 17 138.10 3 597.12 263.18 728.01 200.00 5 000.00 369.84 1 617.98 9 160.70 244.75 地表水 Mean 7.30 301.99 138.71 40.88 8.90 4.43 30.69 85.07 56.11 40.91 23.25 SD 0.32 302.26 127.06 35.80 9.18 5.75 41.82 90.26 62.81 53.38 33.27 Cv 0.04 1.00 0.92 0.88 1.03 1.30 1.36 1.06 1.12 1.30 1.43 Min 6.80 91.69 48.54 15.43 2.31 0.16 4.43 27.98 19.21 7.18 5.52 Max 7.60 814.84 351.78 100.80 24.31 12.59 103.60 243.46 165.70 132.78 82.60 注:Min为最小值,Max为最大值,Mean为平均值,SD为标准差,Cv为变异系数。
Note: Min represents minimum value; Max represents maximum value; Mean represents average value, SD represents standard deviation; Cv represents variation coefficient.表 2 主成分分析法组成矩阵
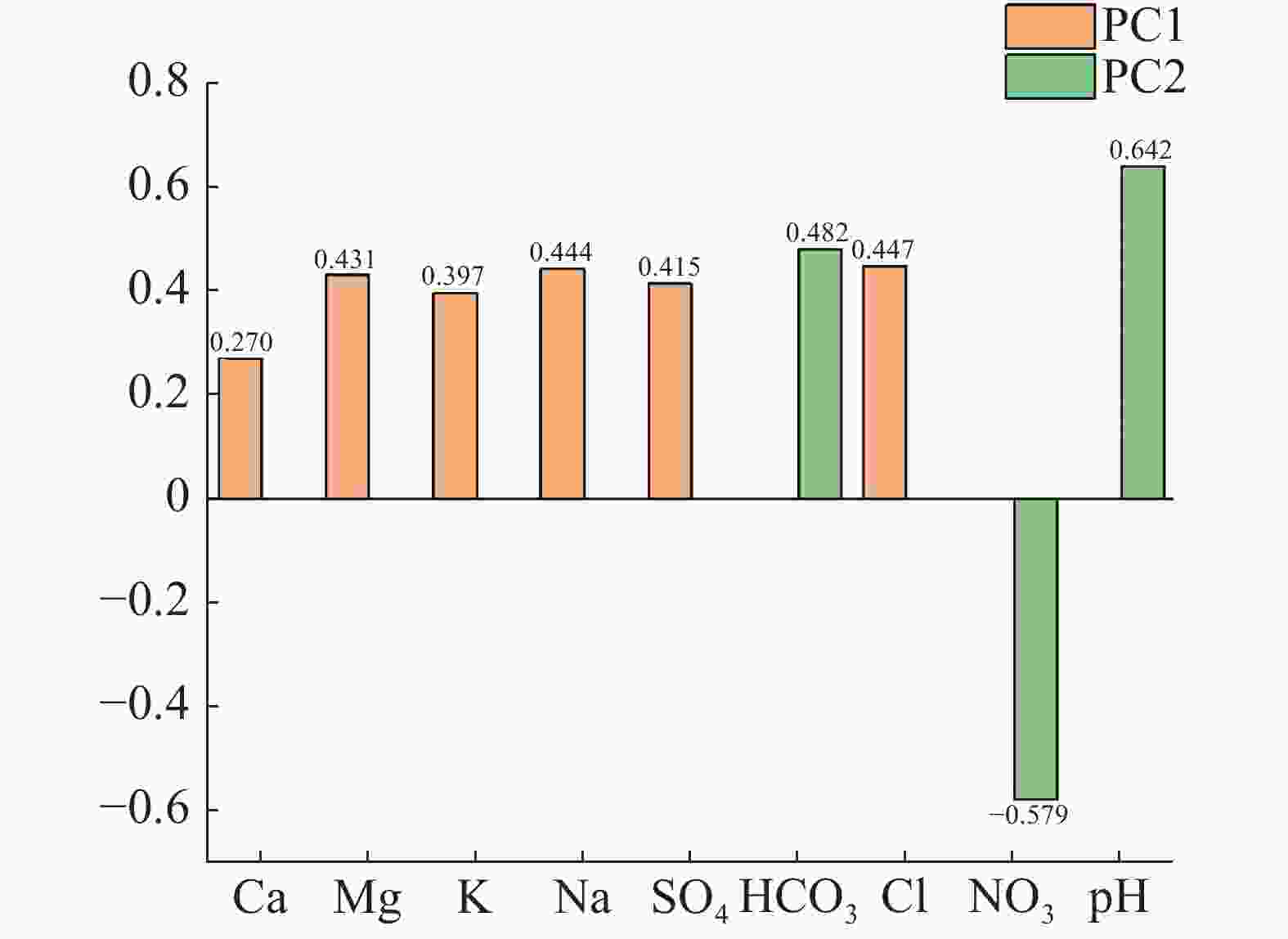
Table 2. Matrix formed by principal component analysis
PC1 PC2 pH 0.007 70 0.641 80 Ca2+ 0.270 27 −0.106 40 Mg2+ 0.431 21 −0.056 97 K+ 0.396 81 −0.004 19 Na+ 0.444 05 0.006 05 ${\rm{HCO}}_3^{-}$ 0.099 17 0.482 26 ${\rm{SO}}_4^{2-}$ 0.414 96 −0.072 80 Cl− 0.447 43 −0.016 36 ${\rm{NO}}_3^{-}$ −0.063 99 −0.579 08 表 3 海水入侵指标的等级划分 (单位/mg·L−1)
Table 3. Classification of indexes of seawater intrusion (unit/mg·L−1)
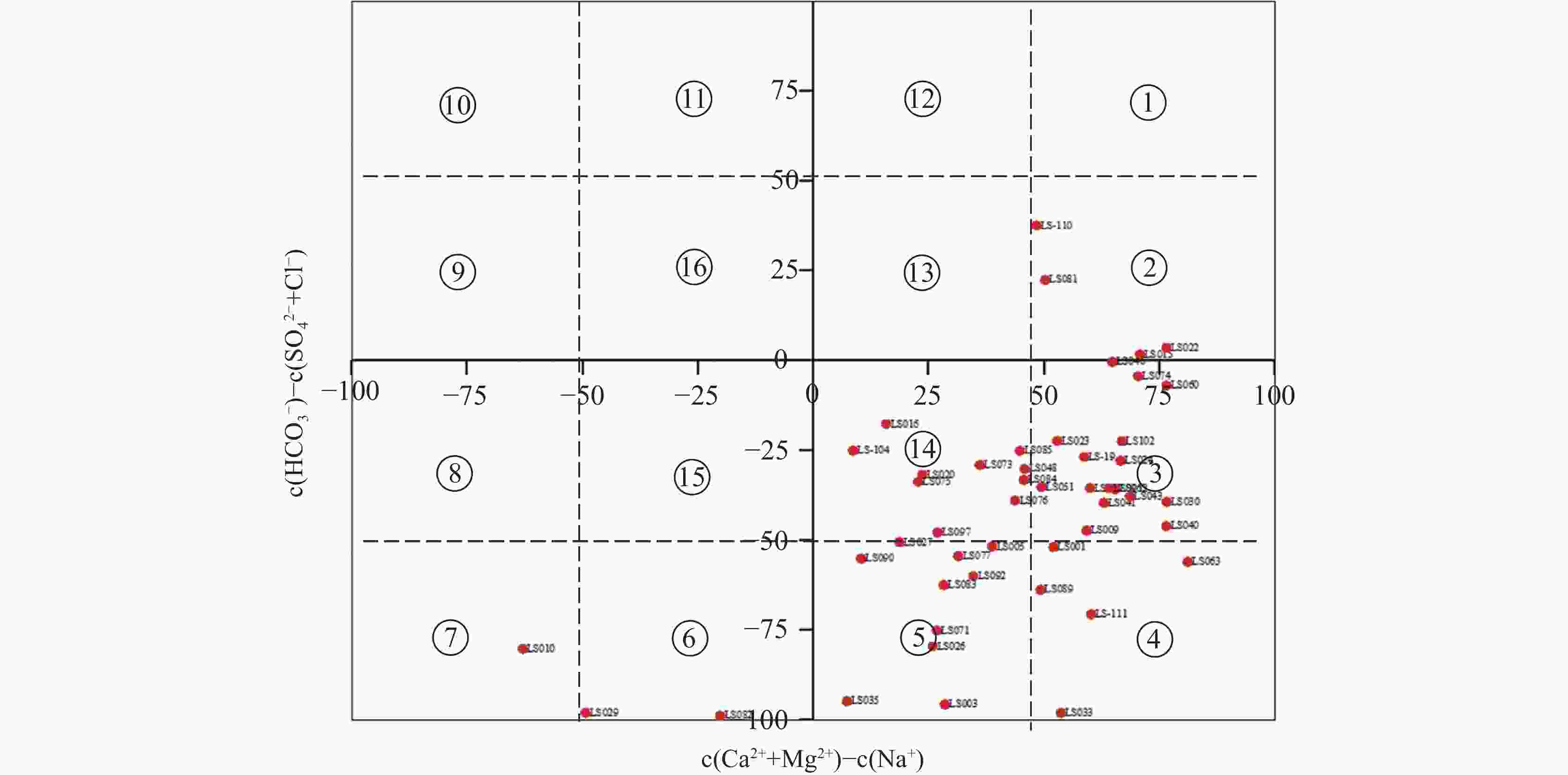
特征因子 I II III IV 无入侵 轻度入侵 中度入侵 严重入侵 Cl− ≤250 ≤600 ≤1 500 >1 500 ${\rm{SO}}_4^{2-}$ ≤200 ≤450 ≤1 200 >1 200 M ≤1 000 ≤2 000 ≤3 000 >3 000 SAR ≤2 ≤3.55 ≤10 >10 γCl/γHCO3 ≤0.5 ≤1.0 ≤6.6 >6.6 -
[1] Andersen M S, Nyvang V, Jakobsen R, Postma D. Geochemical processes and solute transport at the seawater/freshwater interface of a sandy aquifer[J]. Geochimica et Cosmochimica Acta, 2005, 69: 3979–3994. [2] Werner A D, Simmons C T. Impact of sea-level rise on seawater intrusion in coastal aquifer[J]. Groundwater, 2009, 47:197-204. doi: 10.1111/j.1745-6584.2008.00535.x [3] Liu Y, Jiao J J, Liang W, Kuang X. Hydrogeochemical characteristics in coastal groundwater mixing zone[J]. Applied Geochemistry, 2017, 85:49-60. doi: 10.1016/j.apgeochem.2017.09.002 [4] Kazakis N, Pavlou A, Vargemezis G, Voudouris K S, Soulios G, Pliakas F, Tsokas G. Seawater intrusion mapping using electrical resistivity tomography and hydrochemical data. An application in the coastal area of eastern Thermaikos Gulf, Greece[J]. Science of the Total Environment, 2016, 543:373-387. doi: 10.1016/j.scitotenv.2015.11.041 [5] Alfarrah N, Walraevens K. Groundwater overexploitation and seawater intrusion in coastal areas of arid and semi-arid regions[J]. Water, 2018, 10(2):143. [6] Bovolo C I, Parkin G, Sophocleous M. Groundwater resources, climate and vulnerability[J]. Environmental Research Letters, 2009, 4(3):35001. doi: 10.1088/1748-9326/4/3/035001 [7] 郭明华, 王敬, 戴长国, 蒋雷, 张浩清, 柳超. 文登区浅层地下水化学演化与海水入侵研究[J]. 海洋科学, 2021, 45(7):57-65. doi: 10.11759/hykx20200708002GUO Minghua, WANG Jing, DAI Changguo, JIANG Lei, ZHANG Haoqing, LIU Chao. Study on the hydrochemical evolution of groundwater and seawater intrusion in the shallow layer of Wendeng district[J]. Marine Science, 2021, 45(7):57-65. doi: 10.11759/hykx20200708002 [8] 赵倩. 锦州小凌河扇地海水入侵过程中水文地球化学作用及影响因素研究[D]. 廊坊: 防灾科技学院, 2021.ZHAO Qian. Hydrogeochemistry and influencing factors in the process of seawater intrusion in Xiaoling river fan, Jinzhou City[D]. Langfang: Institute of Disaster Prevention, 2021. [9] Mondal NC, Singh VP, Singh VS, Saxena VK. Determining the interaction between groundwater and saline water through groundwater major ions chemistry[J]. Journal of Hydrology, 2010, 388, 100-111. [10] 王昊, 王中良. 天津北大港水库水质咸化的地球化学机理分析[J]. 天津师范大学学报(自然科学版), 2016, 36(6):29-34.WANG Hao, WANG Zhongliang. Geochemical analysis on water salinization mechanism of the Tianjin Beidagang reservoir[J]. Journal of Tianjin Normal University (Natural Science Edition), 2016, 36(6):29-34. [11] 辛祥. 大沽河下游地区地下水氯离子时空变化特征与调控的数值模拟[D]. 青岛: 青岛大学, 2019.XIN Xiang. Numerical simulation of spatial and temporal characteristics and control of chloride ion in groundwater in the downstream area of Dagu river[D]. Qingdao: Qingdao University, 2019. [12] 林曼曼. 青岛近海海域灾害地质特征研究[D]. 石家庄: 石家庄经济学院, 2014.LIN Manman. The study on the characteristics of geo-hazard factors in Qingdao offshore[D]. Shijiazhuang: University of Economics, 2014. [13] 陆求裕. 长乐滨海地区海水入侵现状及成因分析[J]. 地质灾害与环境保护, 2020, 31(4):101-105. doi: 10.3969/j.issn.1006-4362.2020.04.019LU Qiuyu. The present situation of the seawater intrusion along the coastal area of Changle[J]. Journal of Geological Hazards and Environment Preservation, 2020, 31(4):101-105. doi: 10.3969/j.issn.1006-4362.2020.04.019 [14] 梁彬, 关碧珠, 崔光中, 揭江, 唐祚旺. 广东湛江开发巨型盆地防治海水入侵研究[J]. 中国岩溶, 2001, 20(1):77. [15] 秦凯凯. 管状海水入侵形成机理及影响因素研究[D]. 大连: 大连海事大学, 2017.QIN Kaikai. Study on formation mechanism and influencing factors of tubular seawater intrusion[D]. Dalian: Dalian Maritime University, 2017. [16] 崔震, 陈广泉, 徐兴永, 王东亮, 喻龙. 北长山岛海水入侵成因机理及现状评价[J]. 海洋环境科学, 2015, 34(6):930-936. doi: 10.13634/j.cnki.mes.2015.06.021CUI Zhen, CHEN Guangquan, XU Xingyong, WANG Dongliang, YU Long. Mechanism and assessment of seawater intrusion in the northern Changshan island[J]. Marine Environmental Science, 2015, 34(6):930-936. doi: 10.13634/j.cnki.mes.2015.06.021 [17] 徐铭霜. 某沿海地区海水入侵动态变化规律及防治措施研究[D]. 青岛: 青岛理工大学, 2022.XU Mingshuang. Research on the dynamic variation rule of seawater intrusion and controlling measures in a coastal area[D]. Qingdao: Qingdao University of Technology, 2022. [18] 王蜜蕾, 窦衍光, 邹亮, 薛碧颖, 胡睿, 岳保静, 徐刚, 林曦, 李林森. 青岛崂山周边地下水化学特征与矿泉水成因分析[J]. 海洋地质前沿, 2021, 37(9):17-24. doi: 10.16028/j.1009-2722.2021.126WANG Milei, DOU Yanguang, ZOU Liang, XUE Biying, HU Rui, YUE Baojing, XU Gang, LIN Xi, LI Linsen. Hydrochemical characteristics of groundwater and genesis of mineral water at Laoshan mountain and surrounding areas, Qingdao[J]. Marine Geology Frontiers, 2021, 37(9):17-24. doi: 10.16028/j.1009-2722.2021.126 [19] 胡慧君. 青岛市大沽河典型区非饱和−饱和带地下水耦合模型[D]. 南京: 南京大学, 2019.HU Huijun. An integrated modeling of variably saturated groundwater flow system in a typical area of Dagu river basin[D]. Nanjing: Nanjing University, 2019. [20] 刘久潭, 周丹, 高宗军, 王敏, 马媛媛, 张洪英, 时孟杰, 董杰. 青岛西海岸新区地下水水化学特征及水质评价[J]. 山东科技大学学报(自然科学版), 2019, 38(2):14-24, 43. doi: 10.16452/j.cnki.sdkjzk.2019.02.002LIU Jiutan, ZHOU Dan, GAO Zongjun, WANG Min, MA Yuanyuan, ZHANG Hongying, SHI Mengjie, DONG Jie. Hydrochemical characteristics and water quality assessment of groundwater in Qingdao West Coast New District[J]. Journal of Shandong University of Science and Technology (Natural Science), 2019, 38(2):14-24, 43. doi: 10.16452/j.cnki.sdkjzk.2019.02.002 [21] 尹子悦, 林青, 徐绍辉. 青岛市大沽河流域地下水水化学时空演化及影响因素分析[J]. 地质论评, 2018, 64(4):1030-1043. doi: 10.16509/j.georeview.2018.04.017YIN Ziyue, LIN Qing, XU Shaohui. Spatial-temporal variations and controlling factors of groundwater hydrochemical characteristics in the Dagu river basin[J]. Geological Review, 2018, 64(4):1030-1043. doi: 10.16509/j.georeview.2018.04.017 [22] 康晓雨. 青岛市典型富水区地下水环境质量评价及演化规律[D]. 石家庄: 河北地质大学, 2017.KANG Xiaoyu. Groundwater quality evaluation and evolution law of typical rich water area in Qingdao Hebei[D]. Shijiazhuang: Hebei GEO University, 2017. [23] 肖菲, 周勇华, 陈小英. 青岛沿岸海(咸)水入侵灾害的成因与现状[J]. 海洋地质前沿, 2012, 28(5):54-58. doi: 10.16028/j.1009-2722.2012.05.011XIAO Fei, ZHOU Yonghua, CHEN Xiaoying. Research on the origin and current status of sea (salt) water intrusion in coastal areas of Qingdao[J]. Marine Geology Frontiers, 2012, 28(5):54-58. doi: 10.16028/j.1009-2722.2012.05.011 [24] 姜兴钰. 青岛新河地区海水入侵综合研究[D]. 青岛: 国家海洋局第一海洋研究所, 2010. [25] 董少杰, 孟春霞, 王成见. 青岛市地下水源地水质评价及污染原因分析[J]. 水资源与水工程学报, 2006(6):54-57. doi: 10.3969/j.issn.1672-643X.2006.06.013DONG Shaojie, MENG Chunxia, WANG Chengjian. Water quality evaluation and reasons analysis of pollution for groundwater sources in Qingdao[J]. Journal of Water Resources and Water Engineering, 2006(6):54-57. doi: 10.3969/j.issn.1672-643X.2006.06.013 [26] Shaheen A, Iqbal J, Hussain S. Adaptive geospatial modeling of soil contamination by selected heavy metals in the industrial area of Sheikhupura, Pakistan[J]. International Journal of Environmental Science and Technology, 2019, 16(8): 4447-4464. [27] Khan M K, Ayoub W, Saied S, Hussain M M, Masood S S, Siddique A, Khwaja H A. Statistical and geospatial assessment of groundwater quality in the megacity of karachi[J]. Journal of Water Resource and Protection, 2019, 11(3): 311-332. [28] Fatma B B, Emna B, Jalila M, Salem B. Evaluation of groundwater hydrogeochemical characteristics and delineation of geothermal potentialities using multi-criteria decision analysis: Case of Tozeur region, Tunisia[J]. Applied Geochemistry, 2020, 113: 104504. [29] 周爱红, 牛鹏飞, 袁颖, 黄虎城. 基于PCA-PSO-SVM的凡口铅锌矿地区岩溶地表塌陷危险性预测[J]. 中国岩溶, 2020, 39(4):622-628.ZHOU Aihong, NIU Pengfei, YUAN Ying, HUANG Hucheng. Prediction of karst surface subsidence risk in the Fankou lead-zinc mine area based on PCA-PSO-SVM[J]. Carsologica Sinica, 2020, 39(4):622-628. [30] 叶慧君, 张瑞雪, 吴攀, 李学先, 覃应机, 査学芳, 韩志伟. 基于主成分分析的岩溶水水化学组成及影响因素研究: 以贵州水城盆地为例[J]. 中国岩溶, 2017, 36(2): 215-225.YE Huijun, ZHANG Ruixue, WU Pan, LI Xuexian, QIN Yingji, ZHA Xuefang, HAN Zhiwei. Hydrochemical characterization of groundwater and surface water and their influencing factors based on principal component analysis: An example in the Shuicheng basin of Guizhou[J]. Carsologica Sinica, 2017, 36(2): 215-225. [31] 赵艳丽, 王敏, 李常锁. 山东省淄河流域地下水化学污染源解析[J]. 人民长江, 2022, 53(12):37-43, 49. doi: 10.16232/j.cnki.1001-4179.2022.12.006ZHAO Yanli, WANG Min, LI Changsuo. Analysis of chemical pollution source of groundwater in Zihe river basin of Shandong Province[J]. Yangtze River, 2022, 53(12):37-43, 49. doi: 10.16232/j.cnki.1001-4179.2022.12.006 [32] 张洁, 梁杏, 刘延锋, 张鑫, 孙立群, 赵枫, 付鹏宇. 基于主成分的协克里金法对地下水砷空间分布预测[J]. 地球科学, 2023, 48(10): 3820-3831.ZHANG Jie, LIANG Xing, LIU Yanfeng, ZHANG Xin, SUN Liqun, ZHAO Feng, FU Pengyu. The cokriging method is based on principal components to predict the spatial distribution of arsenic in groundwater[J]. Earth Science, 2023, 48(10): 3820-3831. [33] Giménez Forcada E. Dynamic of sea water interface using hydrochemical facies evolution diagram[J]. Groundwater, 2010, 48:212-216. doi: 10.1111/j.1745-6584.2009.00649.x [34] Giménez Forcada E. Space/time development of seawater intrusion: A study case in Vinaroz coastal plain (Eastern Spain) using HFE-Diagram, and spatial distribution of hydrochemical facies[J]. Journal of Hydrology, 2014, 517:617-627. doi: 10.1016/j.jhydrol.2014.05.056 [35] Chadha D K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data[J]. Hydrogeology Journal, 1999(7):431-439. doi: 10.1007/s100400050216 [36] Mondal N C, Singh V P, Singh V S, Saxena V K. Determining the interaction between groundwater and saline water through groundwater major ions chemistry[J]. Journal of Hydrology, 2010, 388:100-111. doi: 10.1016/j.jhydrol.2010.04.032 [37] Nosair A M, Shams M Y, AbouElmagd L M, Hassanein A E, Fryar A E, Abu Salem H S. A predictive model for progressive salinization in a coastal aquifer using artificial intelligence and hydrogeochemical techniques: A case study of the Nile Delta aquifer, Egypt[J]. Environmental Science and Pollution Research, 2022, 29(6):9318-9340. doi: 10.1007/s11356-021-16289-w [38] GB/T 14848-2017. 地下水质量标准[S].GB/T 14848-2017. Standard for Groundwater Quality[S]. [39] Wang Min, Yin Yueping, Wen Dongguang. Handbook of hydrogeology[M]. Beijing: Geology Press, 2012. [40] 寇馨月, 丁军军, 李玉中, 毛丽丽, 李巧珍, 徐春英, 郑欠, 庄姗. 青岛市农区地下水硝态氮污染来源解析[J]. 环境科学, 2021, 42(7): 3232−3241.KOU Xinyue, DING Junjun, LI Yuzhong, MAO Lili, LI Qiaozhen, XU Chunying, ZHENG Qian, ZHUANG Shan. Identifying the sources of groundwater NO3 −-N in agricultural region of Qingdao[J]. Environmental Science, 2021, 42(7): 3232−3241. -




 下载:
下载: