Role of carbonic utilization of microalgae on rock weathering and carbon cycle
-
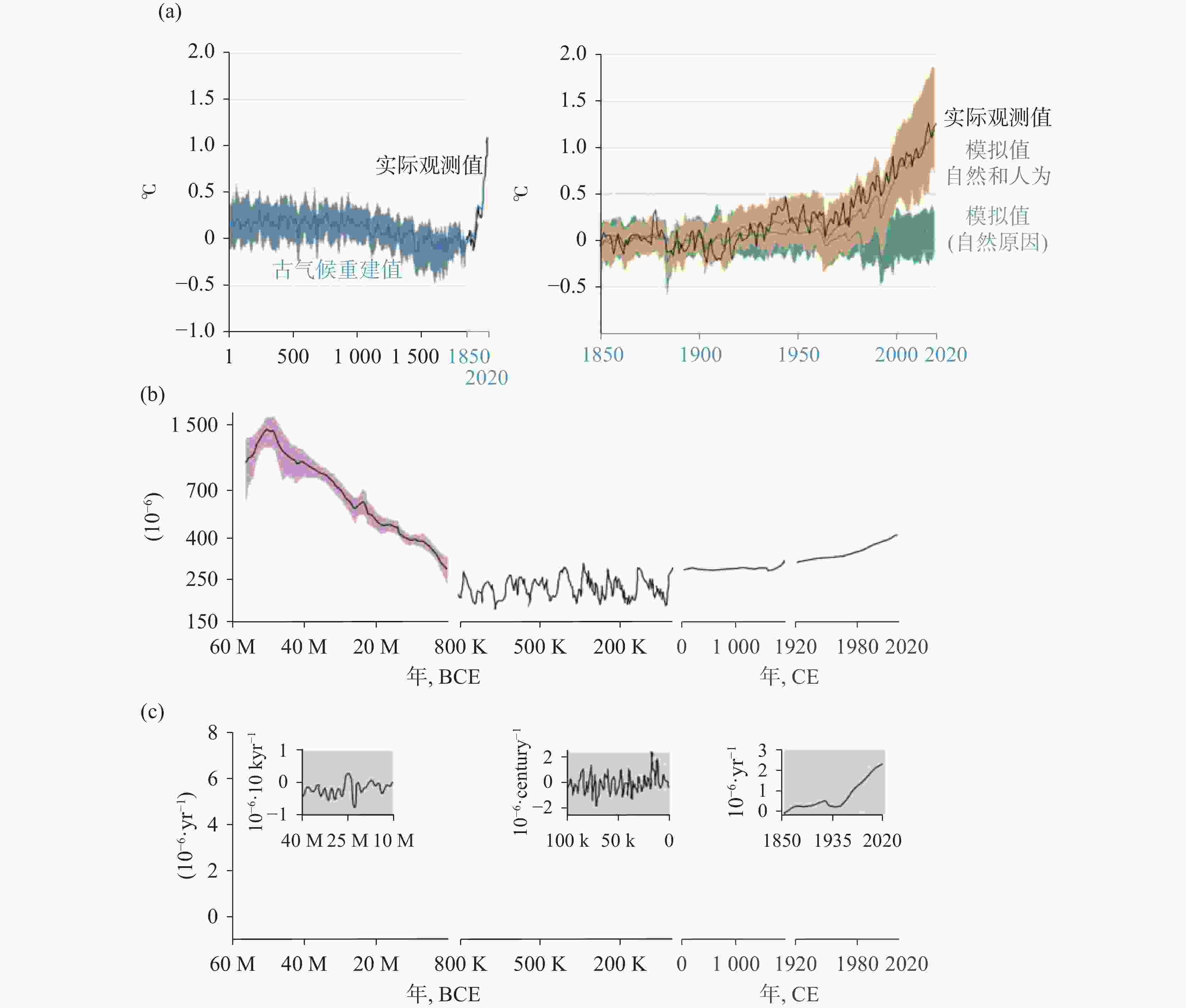
摘要: 岩溶碳汇呈现两种不同观点:(1)岩溶碳汇巨大,其机理在于岩溶区藻类及光合细菌利用碳酸氢根离子(
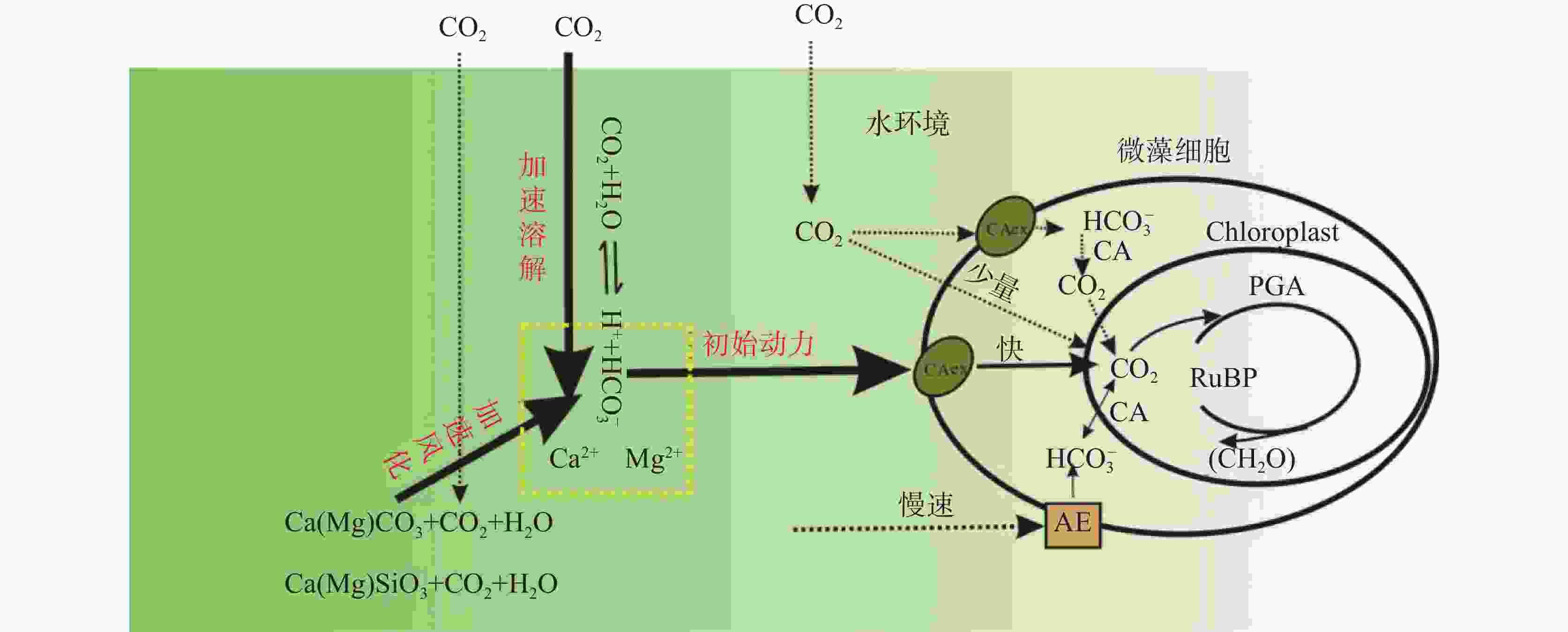
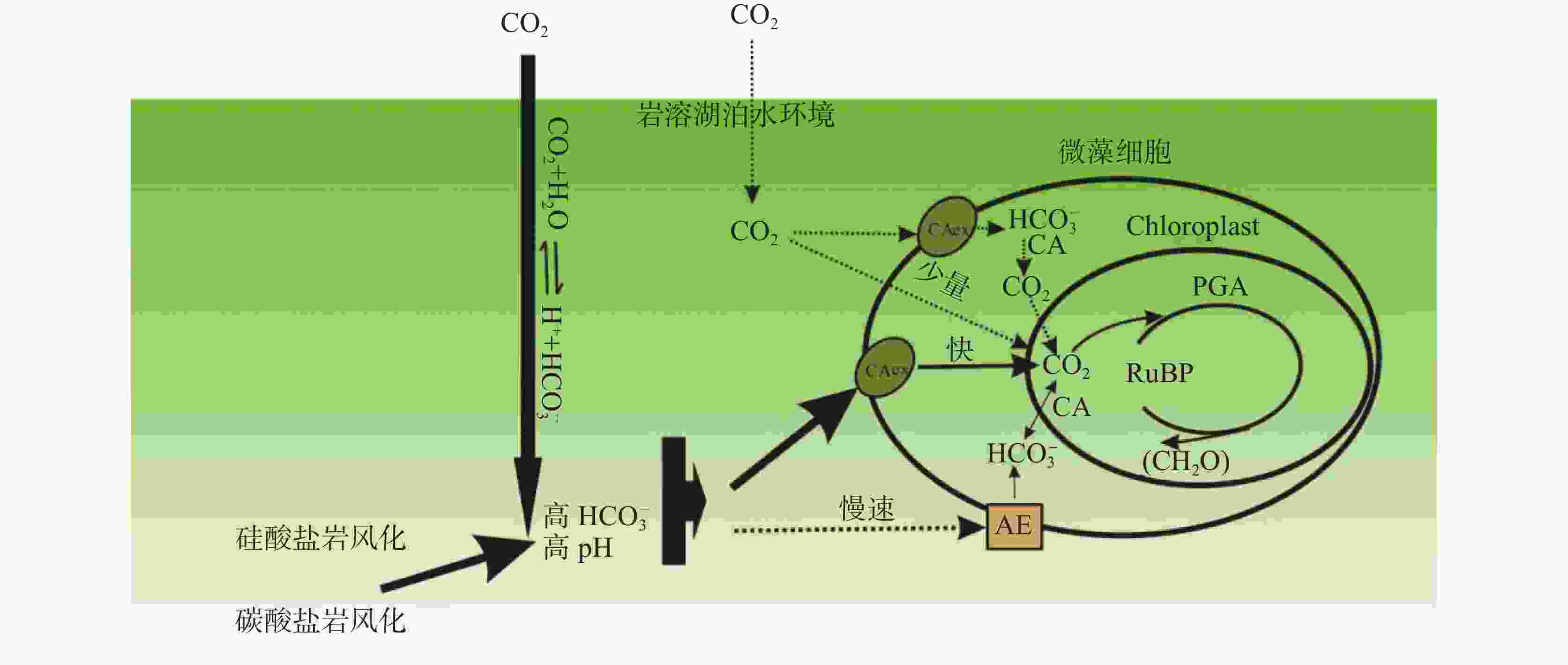
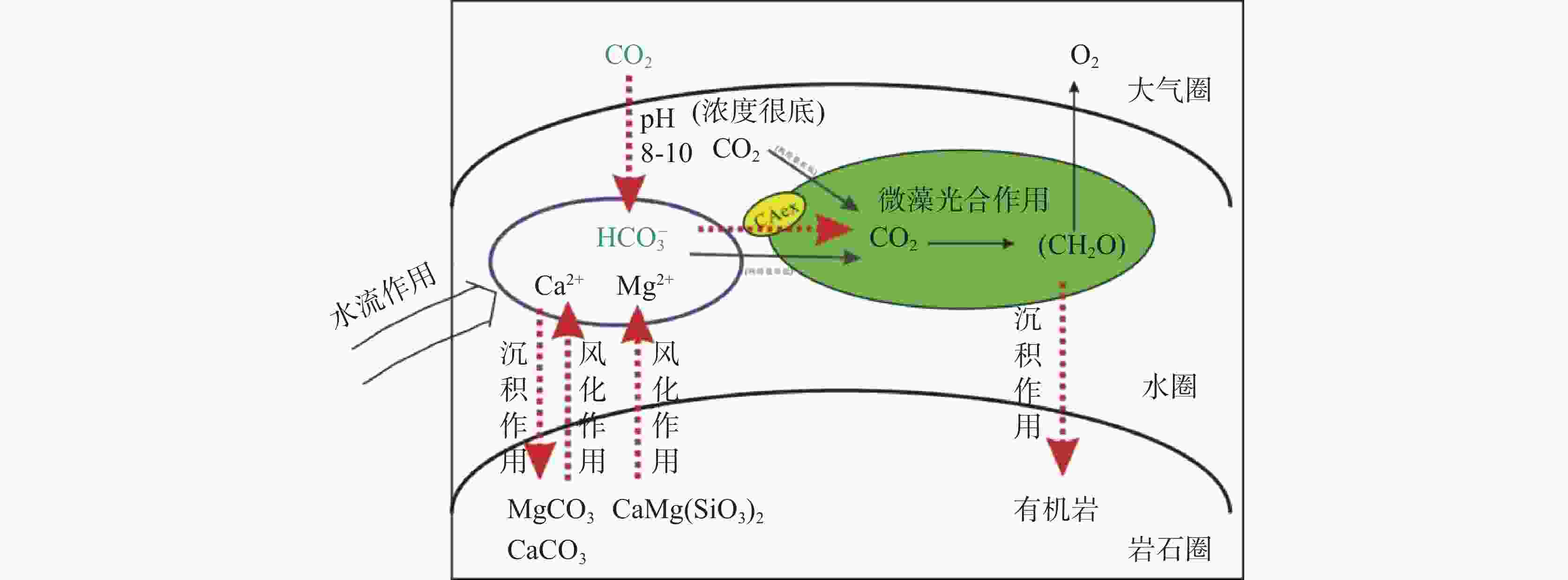
${\rm{HCO}}_3^{-}$ )实现光合作用,从动力学上加速了岩溶风化过程,促进大气CO2的溶解。(2)岩溶区碳酸盐岩的风化作用,产生${\rm{HCO}}_3^{-}$ ,随后产生等量的阳离子在海洋中进行碳酸盐岩的沉积作用,这仅仅体现的是碳酸盐岩的搬运作用,不能体现碳汇,在长期尺度上仅仅有硅酸盐岩风化产生净碳汇。文章抓住岩石风化产生${\rm{HCO}}_3^{-}$ 与微藻光合作用利用${\rm{HCO}}_3^{-}$ 的耦合点,分析了典型代表性水生生物——微藻在无机碳利用上对岩石风化及碳汇的影响。从微藻光合无机碳利用机制以及光合作用关键性酶-碳酸酐酶(CA)作用两方面,论证了微藻生长对岩石风化及其碳汇的的促进作用;同时论述高pH、高${\rm{HCO}}_3^{-}$ 的风化环境对微藻生长影响。获得以下新认识:(1)微藻通过胞外碳酸酐酶(CAex) 利用了大量${\rm{HCO}}_3^{-}$ ,加速岩石风化,并促使风化朝着形成${\rm{HCO}}_3^{-}$ 的方向进行;(2)微藻加速钙镁硅酸盐岩风化,风化溶出的Ca2+、Mg2+会促使碳酸盐岩的沉积,因此微藻加速硅酸盐岩风化形成净碳汇;(3)长时间尺度下,单纯的碳酸盐岩化学风化并不能直接产生净碳汇,但微藻对${\rm{HCO}}_3^{-}$ 利用使得碳酸盐岩风化朝着${\rm{HCO}}_3^{-}$ 转化方向进行,微藻参与碳酸钙沉积作用的同时转化无机碳为惰性有机碳,产生碳汇。故微藻通过CAex的作用,催化加速${\rm{HCO}}_3^{-}$ 与CO2之间的转化,形成水体${\rm{HCO}}_3^{-}$ 消耗的动力基础,微藻无机碳利用对岩石风化具有促进作用,从而调节大气CO2、浓度变化。基于当前研究,提出三点展望:(1)开展岩溶区区域水体系统的岩石风化、水生生物碳汇评估成为解决当前区域碳收支不平衡问题的关键;(2)查明岩石风化作用中生物作用碳转化机理及转化量,解决单纯的水化学径流法计算岩石风化碳汇精度不够问题;(3)构建光合生物参与下的新的评估方法,评估当前岩石风化在水生生物、水循环作用下的碳汇的时间尺度问题,厘清岩石风化碳汇在碳收支中的贡献。-
关键词:
- 微藻 /
- 风化碳汇 /
- ${\rm{HCO}}_3^{-} $ /
- 碳酸酐酶 /
- CO2
Abstract:The carbon sink on rock weathering is widely discussed for reducing global atmospheric carbon dioxide (CO2). Two different views on karst carbon sink are proposed. One is that the karst carbon sink is huge because bicarbonate ion ( ${\rm{HCO}}_3^{-}$ ) is used by the photosynthesis of algae and photosynthetic bacteria in karst areas, which dynamically accelerates the process of karst weathering and subsequently promote the dissolution of atmospheric CO2. Another is that the weathering of carbonate rock generates HCO$_3^{-}$ , and then the equivalent calcium ions (Ca2+) and Magnesium ions (Mg2+) are produced for the deposition of carbonate rock on the sea floor as the river enters the ocean. This process only reflects the transport of carbonate rock instead of the carbon sink because only the weathering of silicate rock may generate the net carbon sink in the long term.By literature review in this paper, the effects of microalgae (a typical aquatic organism) on rock weathering and its carbon sink are discussed based on the coupling between inorganic carbon utilization of microalgae in photosynthesis and ${\rm{HCO}}_3^{-}$ produced from rock-weathering. The facilitation on rock-weathering and its carbon sink by microalgae growth is demonstrated from two aspects, namely, the utilization mechanism of inorganic carbon and the action of carbonic anhydrase (the key enzyme of photosynthesis) in microalgae. Besides, the biomass of microalgae, in turn, is enhanced by the effects of weathering-environment, such as, higher pH value and higher${\rm{HCO}}_3^{-}$ . In this study, the following three arguments are proposed. Firstly, the weathering is accelerated because of the continuous consumption of${\rm{HCO}}_3^{-}$ utilized by catalysis of extracellular carbonic anhydrase (CAex) in microalgae, which makes the weathering towards the direction on forming${\rm{HCO}}_3^{-}$ . Secondly, the microalgae can accelerate the weathering of calcium-magnesium silicate rocks, and Ca2+ and Mg2+ dissolved out by weathering may, in turn, facilitate the deposition of carbonate rock, hence a net carbon sink is generated. Thirdly, pure chemical weathering of carbonate rock cannot directly generate a net carbon sink at long time scale, but the${\rm{HCO}}_3^{-}$ utilization from CO2 in microalgae makes the weathering of carbonate rock proceed in the direction of HCO$_3^{-}$ conversion. In the process of calcium carbonate deposition involved by microalgae, inorganic carbon is converted into recalcitrant organic carbon and thus the carbon sink is generated.Research findings can be concluded that through the CAex effect, the catalysis and acceleration of conversion of HCO $_3^{-}$ to CO2 by microalgae will form the dynamic basis of water HCO$_3^{-}$ consumption. The utilization of inorganic carbon in microalgae can facilitate rock weathering, and hence the concentration of atmospheric CO2 will be regulated. In this study, three aspects of prospect are also put forward. Firstly, to address the regional unbalance of carbon budget, it is crucial to assess the carbon sink of rock weathering under aquatic organism in karst areas. Besides, to improve the precision of calculating carbon sink of rock-weathering by hydrochemical runoff method, the mechanism and amount of biological carbon conversion in rock weathering should be determined. Finally, it is urgent to establish a new method to assess the time scale of carbon sink of rock weathering under the effects of aquatic organisms by water-cycle, which can clarify the contribution of carbon sink of rock weathering to the carbon budget.-
Key words:
- microalgae /
- carbon sink of rock weathering /
- ${\rm{HCO}}_3^{-} $ /
- carbonic anhydrase /
- CO2
-
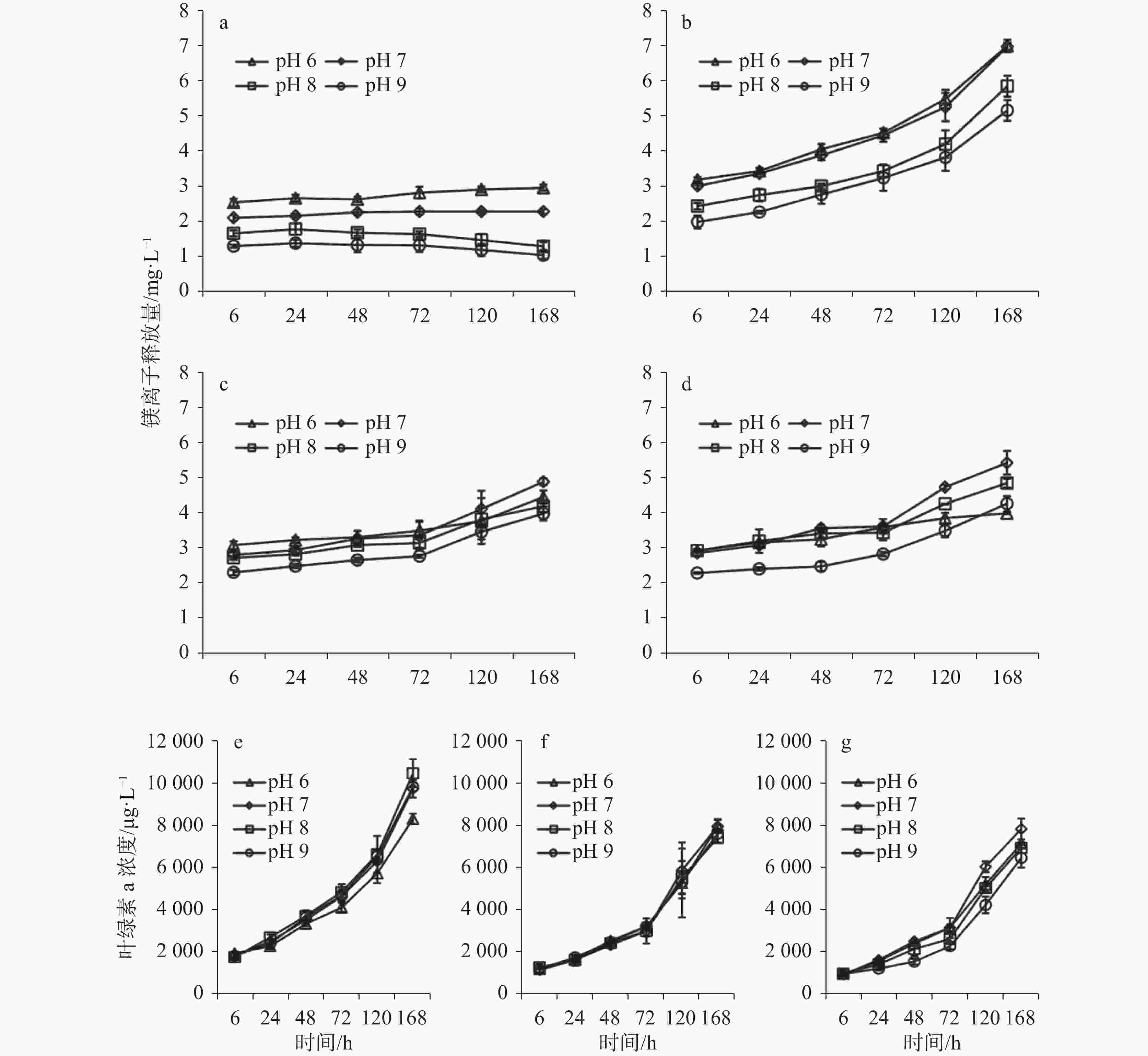
图 5 不同时间下方解石的镁离子释放量(mg·L−1)和叶绿素a的含量(μg·L−1)(a)对照组;(b、e)莱茵衣藻;(c、f)蛋白核小球藻;(d、g)铜绿微囊藻(据Xie Tengxiang等,2017[67])
Figure 5. Concentrations of Mg2+ from calcite (mg·L−1) and chl-a (μg·L−1) at different pH values after different hours of incubation (a) the treatment without microalgae (control group); (b, e) the treatment with C. reinhardtii (C.R.); (c, f) the treatment with C. pyrenoidosa (C.P.); and (d, g) the treatment with M. aeruginosa (M.A.) (revised from Xie Tengxiang et al., 2017[67] )
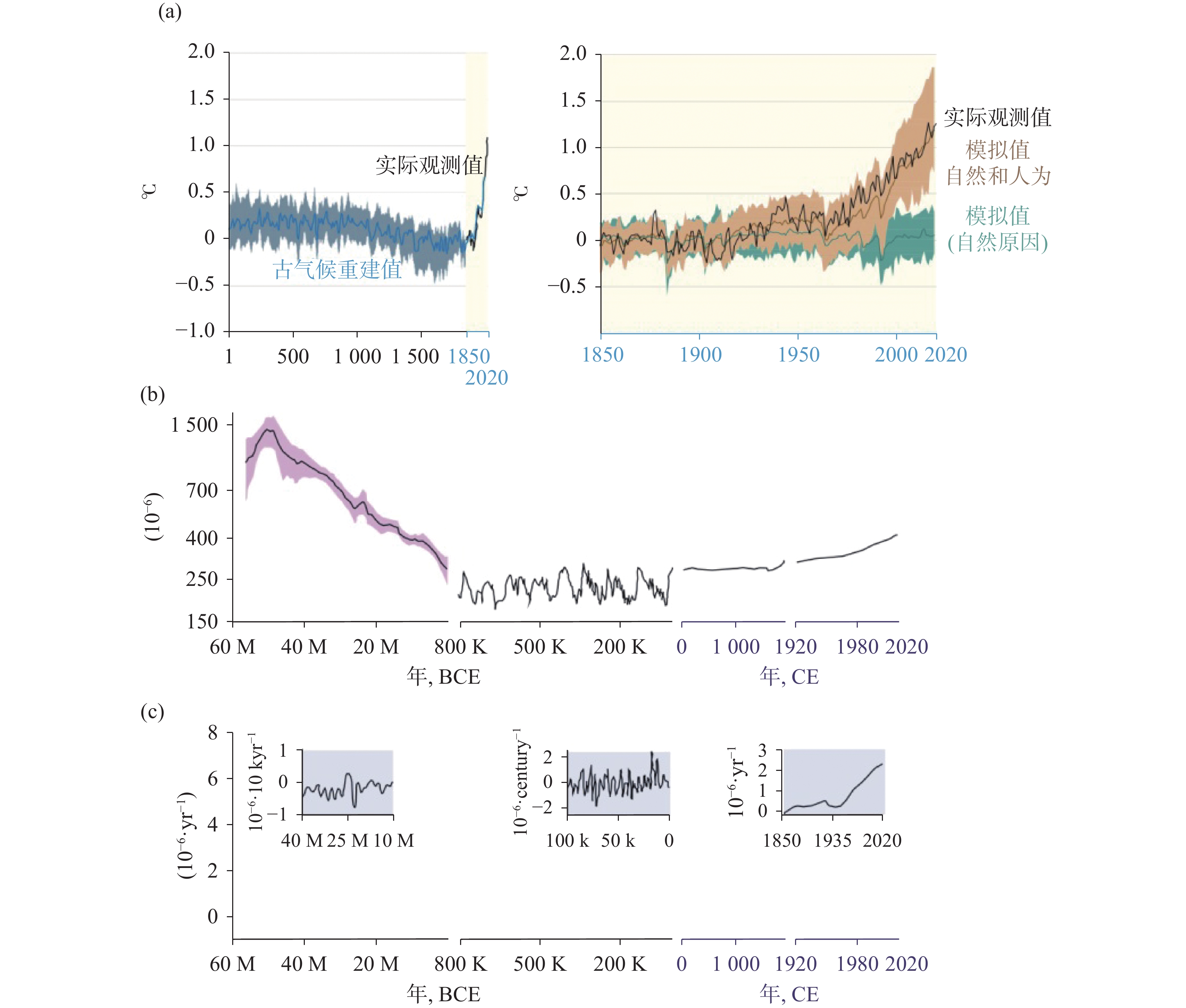
表 1 1850-2019年全球人为累计碳收支情况(引自AR6[1,10])
Table 1. Global accumulated anthropogenic CO2 budget from 1850 to 2019(revised from AR6[1,10])
碳排放量/PgC 总量/PgC 收支不平衡量/PgC 排放(源) 化石燃料燃烧及水泥生产 445±20 685±65 20 净通量土地使用 240±60 分配(汇) 大气增加CO2 265±5 635±80 海洋碳汇 160±20 陆地碳汇 210±55 表 3 微藻的碳酸氢根离子利用途径份额
Table 3. Proportion of bicarbonate utilization pathway to the whole carbon utilization pathway of microalgae
表 4 微藻对碳酸钙碳源的利用份额(fB)(谢腾祥等,2014[104])
Table 4. Proportion of calcium carbonate-carbon source utilized by microalgae (Xie Tengxiang et al., 2014[104])
藻种 AZ/mmol·L−1 fB 莱茵衣藻 0 0.02 0.1 0.03 1 0.08 10 0.30 蛋白核小球藻 0 0.02 0.1 0.02 1 0.10 10 0.15 表 5 野外湖泊微藻利用添加的碳酸氢钠与总无机碳碳源的占比(Li Haitao等,2018[96])
Table 5. Proportion of NaHCO3-carbon source utilized by lake microalgae (Li Haitao et al., 2018[96])
NaHCO3/
mmol·L−1AZ /mmol·L−1 0 1.0 10.0 1.0 0.06±0.03 0.06±0.04 0.03±0.03 2.5 0.08±0.02 0.08±0.05 0.09±0.03 5.0 0.09±0.04 0.17±0.05 0.12±0.05 表 6 碳酸酐酶加速岩石风化的数据统计
Table 6. Statistics of the acceleration of rock weathering by carbonic anhydrase
岩石种类 岩石风化变化情况 生物种类 CA种类 数据来源 白云岩 在CO2分压低于5 000 Pa实验条件下,对白云岩的溶解速率促进倍数在1.29~3.07之间 体外实验 高分子催化剂-牛碳酸酐酶 刘再华,2001[117] 灰岩 加入CA后,在高CO2 分压时,其溶解速率可增加 10倍 体外实验 高分子催化剂-牛碳酸酐酶 刘再华,2001[117] 灰岩 在加入AZ后,单位时间单位藻体的镁离子释放量从3.37×10−4 mg·(μg·day)−1降低至1.99×10−4 mg·(μg·day)−1 莱茵衣藻 胞外碳酸酐酶 Xie等,2014[66] 灰岩 在加入AZ后,单位时间单位藻体的镁离子释放量从2.44×10−4 mg·(μg·day)−1降低至2.19×10−4 mg·(μg·day)−1 蛋白核小球藻 胞外碳酸酐酶 Xie等,2014[66] -
[1] IPCC. Climate Change 2021: The Physical Science Basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change[R]. Cambridge and New York: Cambridge University Press, 2021. [2] IPCC. Climate Change 2013: The Physical Science Basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change[R]. Cambridge and New York: Cambridge University Press, 2013. [3] Foote E. Circumstances affecting the heat of the sun’s rays[J]. American Journal of Science and Arts, 1856: 382–383. [4] Tyndall J. The Bakerian Lecture: On the absorption and radiation of heat by gases and vapours, and on the physical connexion of radiation, absorption, and conduction[J]. Philosophical Transactions of the Royal Society of London, 1861, 151:1-36. doi: 10.1098/rstl.1861.0001 [5] 袁道先, 蒋勇军, 沈立成, 蒲俊兵, 肖琼. 现代岩溶学[M]. 北京: 科学出版社, 2016.YUAN Daoxian, JIANG Yongjun, SHEN Licheng, PU Junbing, XIAO Qiong. Modern karstology[M]. Beijing: Science Press, 2016. [6] Friedlingstein P, Cox P, Betts R, Bopp L, Von Bloh W, Brovkin V, Cadule P, Doney S, Eby M, Fung I, Bala G, John J, Jones C, Joos F, Kato T, Kawamiya M, Knorr W, Lindsay K, Matthews H D, Raddatz T, Rayner P, Reick C, Roeckner E, Schnitzler K G, Schnur R, Strassmann K, Weaver A J, Yoshikawa C, Zeng N. Climate–carbon cycle feedback analysis: Results from the C4MIP model intercomparison[J]. Journal of Climate, 2006, 19(14):3337-3353. doi: 10.1175/JCLI3800.1 [7] Jones C D, Friedlingstein P. Quantifying process-level uncertainty contributions to TCRE and carbon budgets for meeting Paris Agreement climate targets[J]. Environmental Research Letters, 2020, 15(7):1-20. [8] Raupach M R, Gloor M, Sarmiento J L, Canadell J G, Frölicher T L, Gasser T, Houghton R A, QuéréC L, Trudinger C M. The declining uptake rate of atmospheric CO2 by land and ocean sinks[J]. Biogeosciences, 2014, 11(13):3453-3475. doi: 10.5194/bg-11-3453-2014 [9] Williams R G, Katavouta A, Goodwin P. Carbon-cycle feedbacks operating in the climate system[J]. Current Climate Change Reports, 2019, 5(4):282-295. doi: 10.1007/s40641-019-00144-9 [10] Friedlingstein P, O'Sullivan M, Jones M W, Andrew R M, Zaehle S. Global carbon budget 2020[J]. Earth System Science Data, 2020, 12(4):3269-3340. doi: 10.5194/essd-12-3269-2020 [11] Chamberlin T C. A group of hypotheses bearing on climatic changes[J]. Journal of Geology, 1897, 5(7):653-683. doi: 10.1086/607921 [12] Ekholm N. On the variations of the climate of the geological and historical past and their causes[J]. Quarterly Journal of the Royal Meteorological Society, 1901, 27(117):1-62. doi: 10.1002/qj.49702711702 [13] Berner R A. A new look at the long-term carbon cycle[J]. Gsa Today, 1999, 9(11):1-6. [14] Foster G L, Royer D L, Lunt D J. Future climate forcing potentially without precedent in the last 420 million years[J]. Nature Communications, 2017, 8:14845. doi: 10.1038/ncomms14845 [15] Gutjahr M, Ridgwell A, Sexton P F, Anagnostou E, Pearson P N, Pälike H, Norris R D, Thomas E, Foster G L. Very large release of mostly volcanic carbon during the Palaeocene–Eocene Thermal Maximum[J]. Nature, 2017, 548(7669):573-577. doi: 10.1038/nature23646 [16] Raymond P A, Hartmann J, Lauerwald R, Sobek S, McDonald C, Hoover M, Butman D, Striegl R, Mayorga E, Humborg C, Kortelainen P, Dürr H, Meybeck M, Ciais P, Guth P. Global carbon dioxide emissions from inland waters[J]. Nature, 2013, 503(7476):355-359. doi: 10.1038/nature12760 [17] Regnier P, Friedlingstein P, Ciais P, Mackenzie F T, Gruber N, Janssens I A, Laruelle G G, Lauerwald R, Luyssaert S, Andersson A J, Arndt S, Arnosti C, Borges A V, Dale A W, Gallego-Sala A, Goddéris Y, Goossens N, Hartmann J, Heinze C, Ilyina T, Joos F, LaRowe D E, Leifeld J, Meysman F J R, Munhoven G, Raymond P A, Spahni R, Suntharalingam P, Thullner M. Anthropogenic perturbation of the carbon fluxes from land to ocean[J]. Nature Geoscience, 2013, 6(8):597-607. doi: 10.1038/ngeo1830 [18] Lauerwald R, Laruelle G G, Hartmann J, Ciais P, Regnier P A. Spatial patterns in CO2 evasion from the global river network[J]. Global Biogeochemical Cycles, 2015, 29(5):534-554. doi: 10.1002/2014GB004941 [19] Drake J E, Macdonald C A, Tjoelker M G, Reich P B, Singh B K, Anderson I C, Ellsworth D S. Three years of soil respiration in a mature eucalypt woodland exposed to atmospheric CO2 enrichment[J]. Biogeochemistry, 2018, 139(1):85-101. doi: 10.1007/s10533-018-0457-7 [20] Behrenfeld M J. Climate-mediated dance of the plankton[J]. Nature Climate Change, 2014, 4(10):880-887. doi: 10.1038/nclimate2349 [21] Cavicchioli R, Ripple W J, Timmis K N, Azam F, Bakken L R, Baylis M, Behrenfeld M J, Boetius A, Boyd P W, Classen A T, Crowther T W, Danovaro R, Foreman C M, Huisman J, Hutchins D A, Jansson J K, Karl D M, Koskella B, Mark Welch D B, Martiny J B H, Moran M A, Orphan V J, Reay D S, Remais J V, Rich V I, Singh B K, Stein L Y, Stewart F J, Sullivan M B, Van Oppen M J H, Weaver S C, Webb E A, Webster N S. Scientists' warning to humanity: Microorganisms and climate change[J]. Nature Reviews Microbiology, 2019, 17(9):569-586. doi: 10.1038/s41579-019-0222-5 [22] Stone R. The invisible hand behind a vast carbon reservoir[J]. Science, 2010, 328(5985):1476-1477. doi: 10.1126/science.328.5985.1476 [23] Berner R A, Lasaga A C, Garrels R M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years[J]. American Journal of Science, 1983, 283(7):641-683. doi: 10.2475/ajs.283.7.641 [24] 刘再华, Dreybrodt W, 刘洹. 大气 CO2 汇: 硅酸盐风化还是碳酸盐风化的贡献?[J]. 第四纪研究, 2011, 31(3):426-430. doi: 10.3969/j.issn.1001-7410.2011.03.04LIU Zaihua, Dreybrodt W, LIU Huan. Atmospheric CO2 sink: Silicate weathering or carbonate weathering[J]. Quaternary Sciences, 2011, 31(3):426-430. doi: 10.3969/j.issn.1001-7410.2011.03.04 [25] Bricker O P. The global water cycle geochemistry, and environment[J]. Eos, Transactions American Geophysical Union, 1988, 69(4):51-51. [26] Yadav S K, Chakrapani G J, Gupta M K. An experimental study of dissolution kinetics of calcite, dolomite, leucogranite and gneiss in buffered solutions at temperature 25 and 5 C[J]. Environmental Geology, 2008, 53(8):1683-1694. doi: 10.1007/s00254-007-0775-x [27] Wallin M, Bjerle I. A mass transfer model for limestone dissolution from a rotating cylinder[J]. Chemical engineering science, 1989, 44(1):61-67. doi: 10.1016/0009-2509(89)85233-9 [28] Walker J C G, Hays P B, Kasting J F. A negative feedback mechanism for the long‐term stabilization of Earth's surface temperature[J]. Journal of Geophysical Research Oceans, 1981, 86(C10):9776-9782. doi: 10.1029/JC086iC10p09776 [29] 刘再华, W Dreybrodt, 韩军, 李华举. CaCO3-CO2-H2O岩溶系统的平衡化学及其分析[J]. 中国岩溶, 2005, 24(1):3-16. doi: 10.3969/j.issn.1001-4810.2005.01.001LIU Zaihua, Dreybrodt Wolfgang, HAN Jun, LI Huaju. Equilibrium chemistry of the CaCO3-CO2-H2O system and discussions[J]. Carsologica Sinica, 2005, 24(1):3-16. doi: 10.3969/j.issn.1001-4810.2005.01.001 [30] Misra S, Froelich P N. Lithium isotope history of Cenozoic seawater: Changes in silicate weathering and reverse weathering[J]. Science, 2012, 335(6070):818-823. doi: 10.1126/science.1214697 [31] Gaillardet J, Galy A. Atmospheric science. Himalaya-carbon sink or source?[J]. Science, 2008, 320(5884):1727-1728. doi: 10.1126/science.1159279 [32] 徐胜友, 蒋忠诚. 我国岩溶作用与大气温室气体CO2源汇关系的初步估算[J]. 科学通报, 1997, 42(9):953-956. doi: 10.3321/j.issn:0023-074X.1997.09.019XU Shengyou, JIANG Zhongcheng. Preliminary estimation of the relationship between karst and atmospheric greenhouse gas CO2 source and sink in China[J]. Chinese Science Bulletin, 1997, 42(9):953-956. doi: 10.3321/j.issn:0023-074X.1997.09.019 [33] 蒋忠诚, 覃小群, 曹建华, 蒋小珍, 何师意, 罗为群. 中国岩溶作用产生的大气CO2碳汇的分区计算[J]. 中国岩溶, 2011, 30(4):363-367. doi: 10.3969/j.issn.1001-4810.2011.04.002JIANG Zhongcheng, QIN Xiaoqun, CAO Jianhua, JIANG Xiaozhen, HE Shiyi, LUO Weiqun. Calculation of atmospheric CO2 sink formed in karst progresses of the karst divided regions in China[J]. Carsologica Sinica, 2011, 30(4):363-367. doi: 10.3969/j.issn.1001-4810.2011.04.002 [34] 邱冬生, 庄大方, 胡云锋, 姚锐. 中国岩石风化作用所致的碳汇能力估算[J]. 地球科学:中国地质大学学报, 2004, 29(2):177-190.QIU Dongsheng, ZHUANG Dafang, HU Yunfeng, YAO Rui. Estimation of carbon sink capacity caused by rock weathering in China[J]. Earth Science-Journal of China University of Geosciences, 2004, 29(2):177-190. [35] 张之淦. 对《中国岩溶作用产生的大气CO2碳汇的分区计算》一文的商榷[J]. 中国岩溶, 2012, 31(3):339-344. doi: 10.3969/j.issn.1001-4810.2012.03.017ZHANG Zhigan. Discussion on article "Calculation of atmospheric CO2 sink formed in karst processes of karst-divided regions in China"[J]. Carsologica Sinica, 2012, 31(3):339-344. doi: 10.3969/j.issn.1001-4810.2012.03.017 [36] Yuan D X. The carbon cycle in karst[J]. Zeitschrift fur Geomorphologie, 1997, 108(Suppl.):91-102. [37] 刘再华, Wolfgang D, 王海静. 一种由全球水循环产生的可能重要的CO2汇[J]. 科学通报, 2007, 52(20):2418-2422. doi: 10.3321/j.issn:0023-074x.2007.20.013LIU Zaihua, Wolfgang D, WANG Haijing. A potentially important CO2 sink generated by the global water cycle[J]. Chinese Science Bulletin, 2007, 52(20):2418-2422. doi: 10.3321/j.issn:0023-074x.2007.20.013 [38] Fatichi S, Pappas C, Zscheischler J, Leuzinger S. Modelling carbon sources and sinks in terrestrial vegetation[J]. New Phytologist, 2019, 221(2):652-668. doi: 10.1111/nph.15451 [39] Walker A P, De Kauwe M G, Bastos A, Belmecheri S, Georgiou K, Keeling R F, Zuidema P A. Integrating the evidence for a terrestrial carbon sink caused by increasing atmospheric CO2[J]. New Phytologist, 2021, 229(5):2413-2445. doi: 10.1111/nph.16866 [40] Mendonça R, Müller R A, Clow D, Verpoorter C, Raymond P, Tranvik L J, Sobek S. Organic carbon burial in global lakes and reservoirs[J]. Nature communications, 2017, 8(1):1-6. doi: 10.1038/s41467-016-0009-6 [41] Jacobson A R, Mikaloff Fletcher S E, Gruber N, Sarmiento J L, Gloor M. A joint atmosphere-ocean inversion for surface fluxes of carbon dioxide: 1. Methods and global-scale fluxes[J]. Global Biogeochemical Cycles, 2007, 21(1):1-13. [42] Resplandy L, Keeling R F, Rödenbeck C, Stephens B B, Khatiwala S, Rodgers K B, Tans P P. Revision of global carbon fluxes based on a reassessment of oceanic and riverine carbon transport[J]. Nature Geoscience, 2018, 11(7):504-509. doi: 10.1038/s41561-018-0151-3 [43] Bolin B, Degens E T, Kempe S. The global carbon cycle-scope. Carbon in the rock cycle[J]. Amercian Science, 1980, 13:317-342. [44] Garrels R M, Mackenzie F T, Hunt C. Chemical cycles and the global environment: Assessing human influences[J]. La Revue Du Praticien, 1975, 37(23):1321-1325. [45] Kempe S, Degens E T. An early soda ocean?[J]. Chemical geology, 1985, 53(1-2):95-108. doi: 10.1016/0009-2541(85)90023-3 [46] Lenton T M, Britton C. Enhanced carbonate and silicate weathering accelerates recovery from fossil fuel CO2 perturbations[J]. Global Biogeochemical Cycles, 2006, 20, GB3009:1-13. [47] Wallmann K. Controls on the cretaceous and cenozoic evolution of seawater composition, atmospheric CO2 and climate[J]. Geochimica et Cosmochimica Acta, 2001, 65(18):3005-3025. doi: 10.1016/S0016-7037(01)00638-X [48] Caldeira K. Long-term control of atmospheric carbon dioxide; low-temperature seafloor alteration or terrestrial silicate-rock weathering?[J]. American Journal of Science, 1995, 295(9):1077-1114. doi: 10.2475/ajs.295.9.1077 [49] Zhang S, Bai X, Zhao C, Tan Q, Luo G, Wang J, Xi H. Global CO2 consumption by silicate rock chemical weathering: Its past and future[J]. Earth's Future, 2021, 9(5):1-20. [50] Liu C Q, Jiang Y K, Tao F X, Lang Y C, Li S L. Chemical weathering of carbonate rocks by sulfuric acid and the carbon cycling in Southwest China[J]. Geochimica et Cosmochimica Acta, 2008, 4:404-414. [51] Xie C, Gao Q, Tao Z. Reviewed and perspectives of the study on chemical weathering and hydro-chemistry in river basin[J]. Tropical Geography, 2012, 32(4):331-337. [52] Gaillardet J, Dupréb B, Louvata P, Allègrea C J. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers[J]. Chemical Geology, 1999, 159(1-4):3-30. doi: 10.1016/S0009-2541(99)00031-5 [53] Veizer J, Mackenzie F T. Evolution of sedimentary rocks[J]. Treatise on Geochemistry, 2003, 271(1551):369-407. [54] Amiotte Suchet P, Probst J L, Ludwig W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans[J]. Global Biogeochemical Cycles, 2003, 17(2):1038. [55] Hartmann J. Bicarbonate-fluxes and CO2-consumption by chemical weathering on the Japanese Archipelago—application of a multi-lithological model framework[J]. Chemical Geology, 2009, 265(3-4):237-271. doi: 10.1016/j.chemgeo.2009.03.024 [56] Dessert C, Dupré B, François L M, Schott J, Gaillardet J, Chakrapani G, Bajpai S. Erosion of deccan traps determined by river geochemistry: Impact on the global climate and the 87Sr/86Sr ratio of seawater[J]. Earth and Planetary Science Letters, 2001, 188(3-4):459-474. doi: 10.1016/S0012-821X(01)00317-X [57] Berner R A, Kothavala Z. GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic time[J]. American Journal of Science, 2001, 301(2):182-204. doi: 10.2475/ajs.301.2.182 [58] Zhang C, Jiang Z C, He S Y, Jiang Y J, Li L L, Wang J L. The karst dynamic system of vertical zoned climate region: A case study of the Jinfo Mountain state nature reserve in Chongqing[J]. Acta Geoscientica Sinica, 2006(5):510-514. [59] Pu J B, Liu W, Jiang G H, Zhang C. Karst dissolution rate and implication under the impact of rainfall in a typical subtropic karst dynamic system: A Strontium isotope method[J]. Geological Review, 2017, 63:165-176. [60] Liao H, Zhu Y D. Global carbon cycle and climate change in China over the past century[J]. Quaternary Sciences, 2010, 30(3):445-455. [61] Cabré A, Marinov I, Leung S. Consistent global responses of marine ecosystems to future climate change across the IPCC AR5 earth system models[J]. Climate Dynamics, 2015, 45(5):1253-1280. [62] Fu W, Randerson J T, Moore J K. Climate change impacts on net primary production (NPP) and export production (EP) regulated by increasing stratification and phytoplankton community structure in the CMIP5 models[J]. Biogeosciences, 2016, 13(18):5151-5170. doi: 10.5194/bg-13-5151-2016 [63] Flombaum P, Wang W L, Primeau F W, Martiny A C. Martiny, 2020: Global picophytoplankton niche partitioning predicts overall positive response to ocean warming[J]. Nature Geoscience, 2020, 13(2):116-120. doi: 10.1038/s41561-019-0524-2 [64] Wu Y Y, Wang B L, Liu C Q. Chlamydomonas reinhardtii's influence on the corrosion of rocks[C]. First International Conference on Monitoring, Management, Simulation and Remediation of the Geological Environment (Geo-Environment), Segovia, Spain, 2004. WIT Press. [65] Liu Z, Dreybrodt W, Wang H. A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms[J]. Earth-Science Reviews, 2010, 99(3):162-172. [66] Xie T X, Wu Y Y. The role of microalgae and their carbonic anhydrase on the biological dissolution of limestone[J]. Environmental Earth Sciences, 2014, 71(12):5231-5239. doi: 10.1007/s12665-013-2925-7 [67] Xie T X, Wu Y Y. The biokarst system and its carbon sinks in response to pH changes: A simulation experiment with microalgae[J]. Geochemistry, Geophysics, Geosystems, 2017, 18(3):827-843. doi: 10.1002/2016GC006628 [68] 刘再华. 岩石风化碳汇研究的最新进展和展望[J]. 科学通报, 2012, 57(2):95-102.LIU Zaihua. New progress and prospects in the study of rock-weathering-related carbon sinks[J]. Chinese Science Bulletin, 2012, 57(2):95-102. [69] 刘建康. 高级水生生物学[M]. 北京: 科学出版社, 2000: 1-402.LIU Jiankang. Advanced aquatic biology[M]. Beijing: Science Press, 2000: 1-402. [70] Han G, Liu C Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China[J]. Chemical Geology, 2004, 204(1-2):1-21. doi: 10.1016/j.chemgeo.2003.09.009 [71] 刘丛强. 生物地球化学过程与地表物质循环: 西南喀斯特流域侵蚀与生源要素循环[M]. 北京: 科学出版社, 2007.LIU Congqiang. Biogeochemical processes and surface material cycling: Erosion and biomass cycling in a karst basin in Southwest China[M]. Beijing: Science Press, 2007. [72] 周玉婵, 曹建华, 李小方. 水化学对水体着生微型生物群落组成与丰度的影响: 以桂林毛村表层岩溶泉、砂页岩裂隙泉为例[J]. 中国岩溶, 2008, 3:261-265. doi: 10.3969/j.issn.1001-4810.2008.03.011ZHOU Yuchan, CAO Jianhua, LI Xiaofang. Comparison of periphyton community composition and abundance under different hydro-chemical influences between epikarst spring and sand-shale fissure spring in Maocun, Guilin[J]. Carsologica Sinica, 2008, 3:261-265. doi: 10.3969/j.issn.1001-4810.2008.03.011 [73] 王培, 曹建华, 李亮, 杨慧, 李光超. 不同来源小球藻对岩溶水Ca2+, HCO3-利用的初步研究[J]. 水生生物学报, 2013, 37(4):626-631.WANG Pei, CAO Jianhua, LI Liang, YANG Hui, LI Guangchao. Utilization of Ca2+ and HCO3- in karst water by chlorella from different sources[J]. Acta hydrobiologica Sinica, 2013, 37(4):626-631. [74] 王培, 胡清菁, 王朋辉, 李斌, 曹建华. 桂林寨底地下河沉水植物群落结构调查及影响因子分析[J]. 水生态学杂志, 2015, 36(1):34-39.WANG Pei, HU Qingjing, WANG Penghui, LI Bin, CAO Jianhua. Effect of karst geology on the submerged macrophyte community in Zhaidi river, Guilin[J]. Journal of Hydroecology, 2015, 36(1):34-39. [75] Whitfield M, Riley J, Skirrow G. Chemical Oceanography[M]. IP Riley and G. Skirrow (eds. ), 1975: 1-233. [76] Larkum A W, Larkum A W, McComb A J, Shephard S A. Gaseous movement in seagrasses. Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region[M]. Amsterdam: Elsevier Science Publishers BV, 1989, 1(2) : 105-112. [77] 任启飞, 陈椽, 李荔, 王叁, 龙胜兴. 红枫湖秋季浮游植物群落与环境因子关系研究[J]. 环境科学与技术, 2010, 33(12F):59-64.REN Qifei, CHEN Chuan, LI Li, WANG San, LONG Shengxing. Phytoplankton community and its relationship with environmental factors in Hongfeng lake[J]. Environmental Science and Technology, 2010, 33(12F):59-64. [78] 龙胜兴, 陈椽, 郭云, 晏妮, 俞振兴. 红枫湖水库水体富营养化及浮游植物群落结构特征[J]. 中国环境监测, 2013(1):23-29. doi: 10.3969/j.issn.1002-6002.2013.01.005LONG Shengxing, CHEN Chuan, GUO Yun, YAN Ni, YU Zhenxing. Phytoplankton's characteristics of community structure and eutrophication in Hongfeng Lake Reservoir of Guizhou[J]. Environmental Monitoring in China, 2013(1):23-29. doi: 10.3969/j.issn.1002-6002.2013.01.005 [79] 彭希, 刘丛强, 王宝利, 赵颜创, 汪福顺. 河流-水库体系水体表层 pCO2时空变化特征及其扩散通量L以六冲河、洪家渡水库、红枫湖为例[J]. 地球与环境, 2013, 41(2):4-10.PENG Xi, LIU Congqiang, WANG Baoli, ZHAO Yanchuang, WANG Fushun. Spatiotemporal characteristics and diffusion flux of partial pressure of dissolved carbon dioxide (pCO2) in the river-reservoir system as exemplified by the Liuchonghe river, Hongjiadu reservoir and Hongfenghu lake[J]. Earth and Environment, 2013, 41(2):4-10. [80] Emerson R, Green L. Manometric measurements of photosynthesis in the marine alga Gigartina[J]. The Journal of General Physiology, 1934, 17(6):817-842. doi: 10.1085/jgp.17.6.817 [81] Tseng C K, Sweeney B M. Physiological studies of Gelidium cartilagineum. I. Photosynthesis, with special reference to the carbon dioxide factor[J]. American Journal of Botany, 1946, 33(9):706-715. doi: 10.1002/j.1537-2197.1946.tb12929.x [82] Badger M R, Andrews T J, Whitney S M, Ludwig M, Yellowlees D C, Leggat W, Price G D. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae[J]. Canadian Journal of Botany, 1998, 76(6):1052-1071. doi: 10.1139/b98-074 [83] Badger M R, Hanson D, Price G D. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria[J]. Functional Plant Biology, 2002, 29(3):161-173. doi: 10.1071/PP01213 [84] Bozzo G G, Colman B. The induction of inorganic carbon transport and external carbonic anhydrase in Chlamydomonas reinhardtii is regulated by external CO2 concentration[J]. Plant, Cell & Environment, 2000, 23(10):1137-1144. [85] Badger M R, Price G D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution[J]. Journal of Experimental Botany, 2003, 54(383):609-622. doi: 10.1093/jxb/erg076 [86] Giordano M, Beardall J, Raven J A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution[J]. Annual Reviews of Plant Biology, 2005, 56:99-131. doi: 10.1146/annurev.arplant.56.032604.144052 [87] Sültemeyer D. Carbonic anhydrase in eukaryotic algae: Characterization, regulation, and possible function during photosynthesis[J]. Canadian Journal of Botany, 1998, 76(6):962-972. doi: 10.1139/b98-082 [88] Khalifah R G. The carbon dioxide hydration activity of carbonic anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C[J]. Journal of Biological Chemistry, 1971, 246(8):2561-2573. doi: 10.1016/S0021-9258(18)62326-9 [89] Fukuzawa H, Suzuki E, Komukai Y, Miyachi S. A gene homologous to chloroplast carbonic anhydrase (icfA) is essential to photosynthetic carbon dioxide fixation by Synechococcus PCC7942[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(10):4437-4441. doi: 10.1073/pnas.89.10.4437 [90] Funke R P, Kovar J L, Weeks D P. Intracellular carbonic anhydrase is essential to photosynthesis in chlamydomonas reinhardtii at atmospheric levels of CO2 (demonstration via genomic complementation of the high-CO2-requiring mutant Ca-1)[J]. Plant Physiology, 1997, 114(1):237-244. doi: 10.1104/pp.114.1.237 [91] McNevin D B, Badger M R, Whitney S M, Von Caemmerer S, Tcherkez G G, Farquhar G D. Differences in carbon isotope discrimination of three variants of d-ribulose-1, 5-bisphosphate carboxylase/oxygenase reflect differences in their catalytic mechanisms[J]. Journal of Biological Chemistry, 2007, 282(49):36068-36076. doi: 10.1074/jbc.M706274200 [92] Tsuzuki M, Miyachi S. The function of carbonic anhydrase in aquatic photosynthesis[J]. Aquatic Botany, 1989, 34(1):85-104. [93] Sharkia R, Beer S, Cabantchik Z I. A membrane-located polypeptide of Ulva sp. which may be involved in HCO3- uptake is recognized by antibodies raised against the human red-blood-cell anion-exchange protein[J]. Planta, 1994, 194(2):247-249. doi: 10.1007/BF01101684 [94] Invers O, Pérez M, Romero J. Bicarbonate utilization in seagrass photosynthesis: Role of carbonic anhydrase in Posidonia oceanica (L.) Delile and Cymodocea nodosa (Ucria) Ascherson[J]. Journal of Experimental Marine Biology and Ecology, 1999, 235(1):125-133. doi: 10.1016/S0022-0981(98)00172-5 [95] 吴沿友. 植物碳酸酐酶对稳定碳同位素分馏作用的影响[J]. 矿物岩石地球化学通报, 2008, 2:175-179. doi: 10.3969/j.issn.1007-2802.2008.02.011WU Yanyou. The effect of plant carbonic anhydrase on the fractionation of stable carbon isotope[J]. Bulletin of Mineralogy‚ Petrology and Geochemistry, 2008, 2:175-179. doi: 10.3969/j.issn.1007-2802.2008.02.011 [96] Li H T, Wu Y Y, Zhao L H. Effects of carbon anhydrase on utilization of bicarbonate in microalgae: A case study in Lake Hongfeng[J]. Acta Geochimica, 2018, 37(4):519-525. doi: 10.1007/s11631-018-0277-4 [97] Moroney J V, Tolbert N E. Inorganic carbon uptake by Chlamydomonas reinhardtii[J]. Plant Physiology, 1985, 77(2):253-258. doi: 10.1104/pp.77.2.253 [98] 赵丽华, 吴沿友, 谢腾祥, 李海涛, 张开艳, 杭洪涛. 微藻CO2同化过程的稳定碳同位素分馏值[J]. 中国岩溶, 2016, 35(4):357-362.ZHAO Lihua, WU Yanyou, XIE Tengxiang, LI Haitao, ZHANG Kaiyan, HANG Hongtao. Stable carbon isotope fraction (δ13C) of microalgae on CO2 assimilation[J]. Carsologica Sinica, 2016, 35(4):357-362. [99] Wu Y Y. Bicarbonate use and carbon dioxide concentrating mechanisms in photosynthetic organisms[J]. Acta Geochimica, 2021, 40:846-853. doi: 10.1007/s11631-021-00488-w [100] Wu Y Y, Li H T, Xie T X. The regulation on carbon source and carbon sequestration by microalgal carbonic anhydrase. Biogeochemical action of microalgal carbonic anhydrase[M]. Beijing: Science Press, 2015: 76–111. [101] 赵丽华. 两种微藻对橄榄岩的生物溶蚀作用及其碳汇效应[D]. 贵阳: 中国科学院地球化学研究所, 2017.ZHAO Lihua. Biological dissolution of the two kinds of microalgae on the peridotite and the carbon sink[D]. Guiyang: Institute of Geochemistry, Chinese Academy of Sciences, 2017. [102] 吴沿友, 邢德科, 刘莹. 植物利用碳酸氢根离子的特征分析[J]. 地球与环境, 2011, 39(2):273-277.WU Yanyou‚ XING Deke‚ LIU Ying. The characteristics of bicarbonate used by plants[J]. Earth and Environment, 2011, 39(2):273-277. [103] 吴沿友, 李海涛, 谢腾祥. 微藻碳酸酐酶生物地球化学作用[M]. 北京: 科学出版社, 2015.WU Yanyou, LI Haitao, XIE Tengxiang. Biogeochemistry of carbonic anhydrase in microalgae[M]. Beijing: Science Press, 2015. [104] 谢腾祥, 吴沿友. 碳酸钙中的碳能被微藻利用吗[J]. 地球与环境, 2014, 42(2):168-173.XIE Tengxiang, WU Yanyou. The characteristics of bicarbonate used by plants[J]. Earth and Environment, 2014, 42(2):168-173. [105] Aizawa, K. Carbonic anhydrase and CO2 concentrating mechanisms in microalgae and cyanobacteria[J]. Fems Microbiology Letters, 1986, 39(3):215-233. doi: 10.1111/j.1574-6968.1986.tb01860.x [106] Badger M R, Price G D. The CO2 concentrating mechanism in cyanobacteria and microalgae[J]. Physiologia Plantarum, 1992, 84:606-615. doi: 10.1111/j.1399-3054.1992.tb04711.x [107] Pesheva I, Kodama M, Dionisio-Sese M L. Changes in photosynthetic characteristics induced by transferring air-grown cells of Chlorococcum littorale to high-CO2 conditions.[J]. Plant and Cell Physiology, 1994, 35(3):379-387. [108] Sülteméyer D, Schmidt C, Fock H P. Carbonic anhydrase in higher plants and aquatic microorganisms[J]. Physiologia Plantarum, 1995, 88(1):179-190. [109] Suzuki K, Ikawa T. Oxygen enhancement of photosynthetic 14CO2 fixation in a freshwater diatom Nitzschia ruttneri[J]. The Japanese Journal of Phycology, 1993, 41:19-28. [110] Williams T G, Colman B. Rapid separation of carbonic anhydrase isozymes using cellulose acetate membrane electrophoresis[J]. Journal of Experimental Botany, 1994, 45:153-158. doi: 10.1093/jxb/45.1.153 [111] Williams T G, Colman B. Quantification of the contribution of CO2, HCO3-, and external carbonic anhydrase to photosynthesis at low dissolved inorganic carbon in Chlorella saccharophila[J]. Plant Physiology, 1995, 107(1):245-251. doi: 10.1104/pp.107.1.245 [112] Fujiwara S, Fukuzawa H, Tachiki A, Miyachi S. Structure and differential expression of two genes encoding carbonic anhydrase in Chlamydomonas reinhardtii[J]. Proceedings of the National Academy of Sciences of the United States of America, 1990, 87(24):9779-9783. doi: 10.1073/pnas.87.24.9779 [113] Fukuzawa H, Fujiwara S, Tachiki A, Miyachi S. Nucleotide sequences of 2 genes CAH1 and CAH2 which encode carbonic anhydrase polypeptides in Chlamydomonas reinhardtii[J]. Nucleic Acids Research, 1990, 18(21):6441-6442. doi: 10.1093/nar/18.21.6441 [114] Moroney J V, Ma Y, Frey W D, Fusilier K A, Pham T T, Simms T A, Mukherjee B. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles[J]. Photosynthesis Research, 2011, 109(1):133-149. [115] Yoshioka S, Taniguchi F, Miura K, Inoue T, Yamano T, Fukuzawa H. The novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1, encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii[J]. The Plant Cell, 2004, 16(6): 1466-1477. [116] Badger M R, Price G D. The role of carbonic anhydrase in photosynthesis[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1994, 45:369-392. doi: 10.1146/annurev.pp.45.060194.002101 [117] 刘再华. 碳酸酐酶对碳酸盐岩溶解的催化作用及其在大气CO2沉降中的意义[J]. 地球学报, 2001, 22(5):477-480. doi: 10.3321/j.issn:1006-3021.2001.05.018LIU Zaihua. The role of carbonic anhydrase as an activator in carbonate rock dissolution and its significance in atmospheric CO2 precipitation[J]. Acta Geoscientia Sinica, 2001, 22(5):477-480. doi: 10.3321/j.issn:1006-3021.2001.05.018 [118] 刘再华, Dreybrodt W. 不同CO2分压条件下的白云岩溶解动力学机理[J]. 中国科学(B辑化学), 2001, 31(4):377-384.LIU Zaihua, Dreybrodt W. Kinetic mechanism of dolomite dissolution under different CO2 partial pressures[J]. Science in China (Series B), 2001, 31(4):377-384. [119] 曾宪东. 植物碳酸酐酶对石灰岩岩溶的驱动作用研究[D]. 武汉: 华中科技大学, 2004.Zeng Xiandong. Study on driving effect of plant carbonic anhydrase on dissolution of the limestone[D]. Wuhan: Huazhong University of Science & Technology, 2004. [120] 余龙江, 吴云, 李为, 曾宪东, 付春华. 微生物碳酸酐酶对石灰岩的溶蚀驱动作用研究[J]. 中国岩溶, 2004, 23(3):225-228. doi: 10.3969/j.issn.1001-4810.2004.03.008YU Longjiang, WU Yun, LI Wei, ZENG Xiandong, FU Chunhua. Study on the driving effects on limestone corrosion by microbial carbonic anhydrase[J]. Carsologica Sinica, 2004, 23(3):225-228. doi: 10.3969/j.issn.1001-4810.2004.03.008 [121] 申泰铭, 邢必果, 李为, 余龙江. 不同种类微生物及其碳酸酐酶对CO2-H2O-碳酸盐系统中碳酸盐岩的溶蚀作用[J]. 矿物岩石地球化学通报, 2014, 33(6):797-800. doi: 10.3969/j.issn.1007-2802.2014.06.007SHEN Taiming, XIN Biguo, LI Wei, YU Longjiang. Characteristics of carbonate rock corrosion by different kinds of microbes and their carbonic anhydras in the CO2-H2O-Carbonate system[J]. Bulletin of Mineralogy‚ Petrology and Geochemistry, 2014, 33(6):797-800. doi: 10.3969/j.issn.1007-2802.2014.06.007 [122] Xiao B, Lian B, Sun L, Shao W. Gene transcription response to weathering of K-bearing minerals by Aspergillus fumigatus[J]. Chemical Geology, 2012, 306:1-9. [123] Xiao L, Lian B. Heterologously expressed carbonic anhydrase from Bacillus mucilaginosus promoting CaCO3 formation by capturing atmospheric CO2[J]. Carbonates Evaporites, 2015, 31:39-45. [124] Xiao L, Lian B, Hao J, Liu C, Wang S. Effect of carbonic anhydrase on silicate weathering and carbonate formation at present day CO2 concentrations compared to primordial values[J]. Scientific Reports, 2015, 5: 7733. [125] 孙蕾蕾, 肖雷雷, 肖波, 王伟英, 潘辰, 王世杰, 连宾. 黑曲霉风化含钾矿石过程中碳酸酐酶和半胱氨酸合成酶基因表达量的差异[J]. 中国科学:地球科学, 2013, 11:1828-1833.SUN Leilei, XIAO Leilei, XIAO Bo, WANG Weiying, PAN Chen, WANG Shijie, LIAN Bin. Differences in the gene expressive quantities of carbonic anhydrase and cysteine synthase in the weathering of potassium-bearing minerals by Aspergillus niger[J]. Science China: Earth Sciences, 2013, 11:1828-1833. [126] WU Yanyou, LI Pingping, WANG Baoli, LIU Congqiang. Variation in trophic status of three karst reservoirs in the Yunnan-Guizhou Plateau in China[J]. Cryptogamie Algologie, 2008, 29(1):81-93. [127] 谢腾祥. 微藻对碳酸盐矿物的生物溶蚀和沉淀作用及其碳汇效应[D]. 贵阳: 中国科学院地球化学研究所, 2014.XIE Tengxiang. Microalgae's biological dissolution and precipitation on carbonate minerals and carbon sink[D]. Guiyang: Institute of Geochemistry, Chinese Academy of Sciences, 2014. [128] 李海涛. 碳酸酐酶对微藻碳汇组成的影响[D]. 贵阳. 中国科学院地球化学研究所, 2013.LI Haitao. The effect of carbonic anhydrase in microalgae on carbon sequestion[D]. Guiyang: Institute of Geochemistry, Chinese Academy of Sciences, 2013. [129] 李海涛, 吴沿友, 谢腾祥. 微藻利用不同无机碳途径的定量方法[J]. 地球与环境, 2014, 42(1):116-121.LI Haitao, WU Yanyou, XIE Tengxiang. The method of quantifying inorganic carbon pathways in microalgae[J]. Earth and Environment, 2014, 42(1):116-121. [130] Sunderhaus S, Dudkina N V, Jansch L, Klodmann J, Heinemeyer J, Perales M, Zabaleta E, Boekema E J, Braun H P. Carbonic anhydrase subunits form a matrix-exposed domain attached to the membrane arm of mitochondrial complex I in plants[J]. Journal of Biological Chemistry, 2006, 281(10):6482-6488. doi: 10.1074/jbc.M511542200 -







 下载:
下载: