Influence process of thermal structure variations of a karst water reservoir on dissolved inorganic carbon and its stable carbon isotope
-
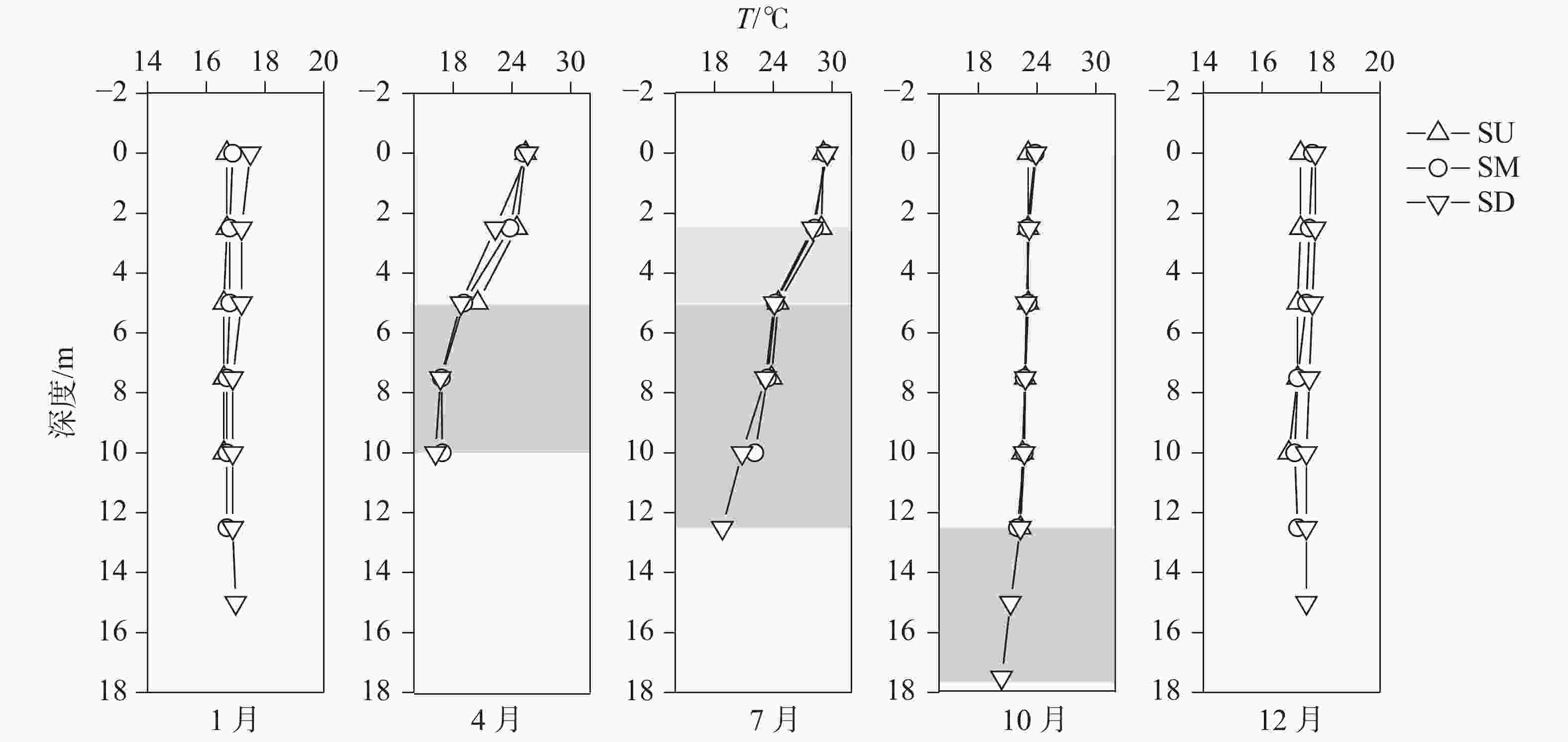
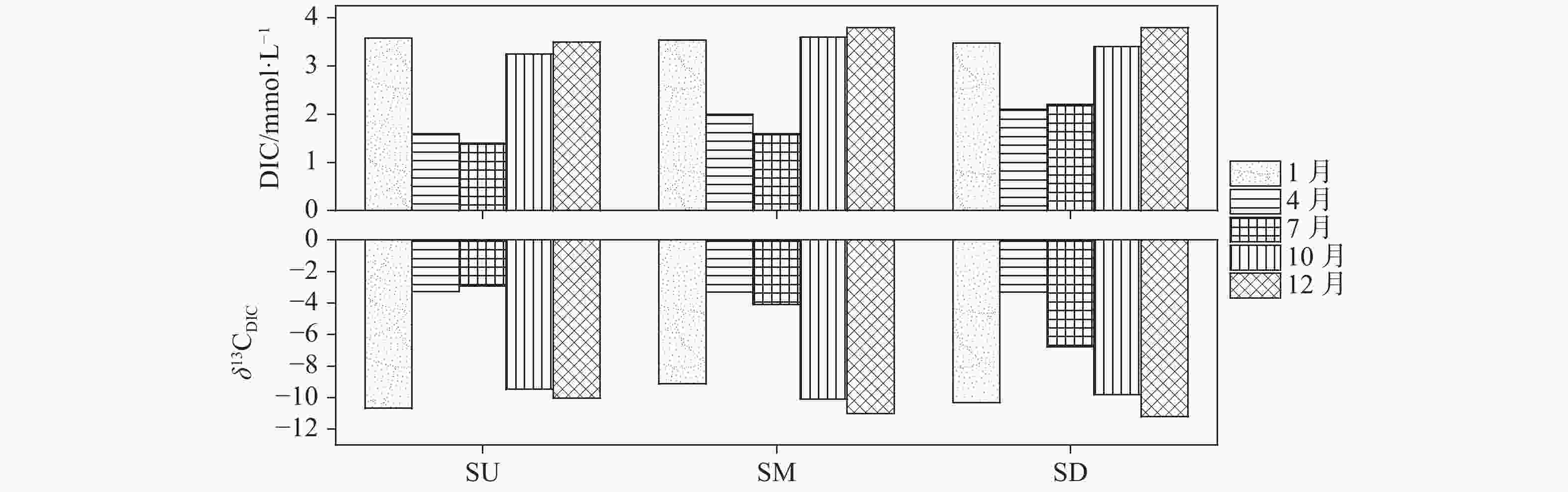
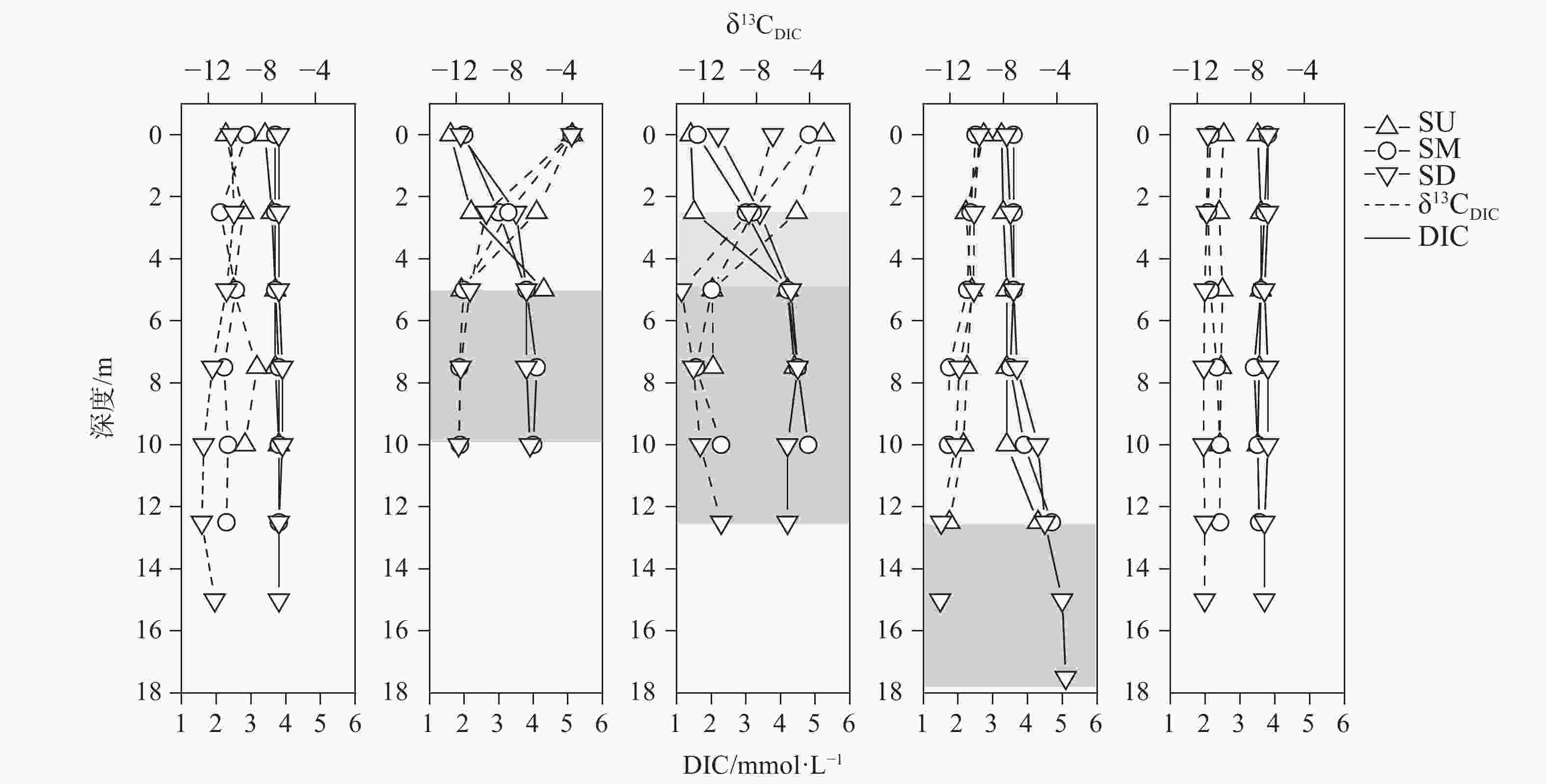
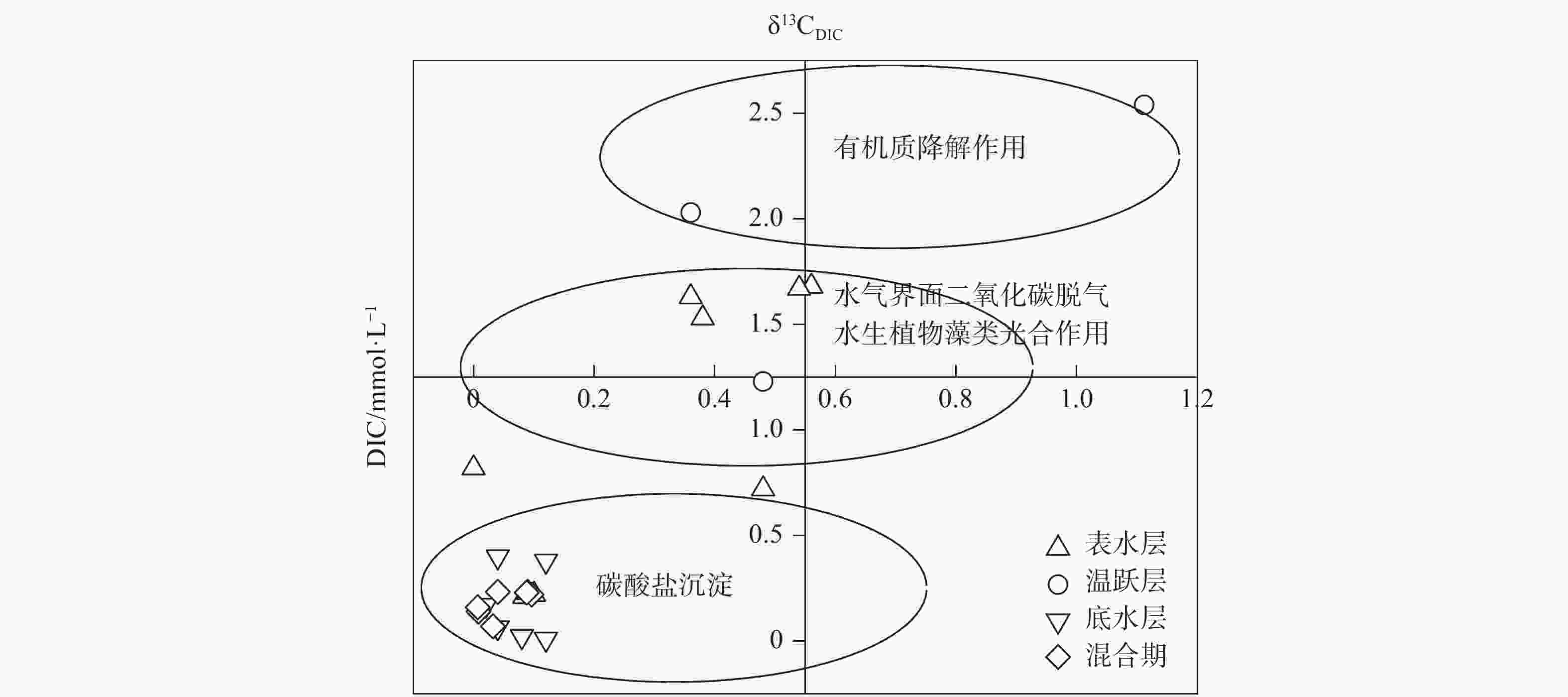
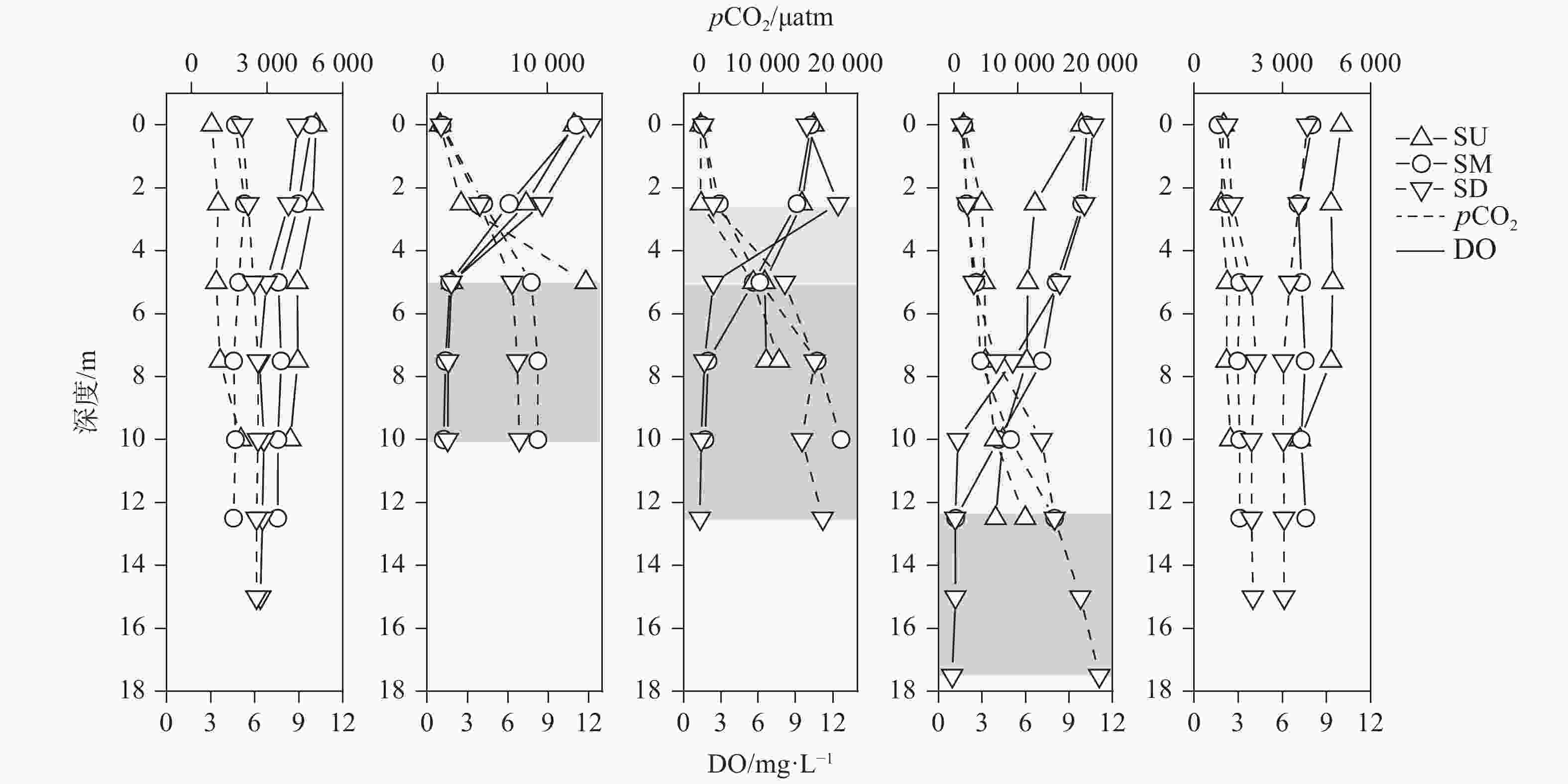
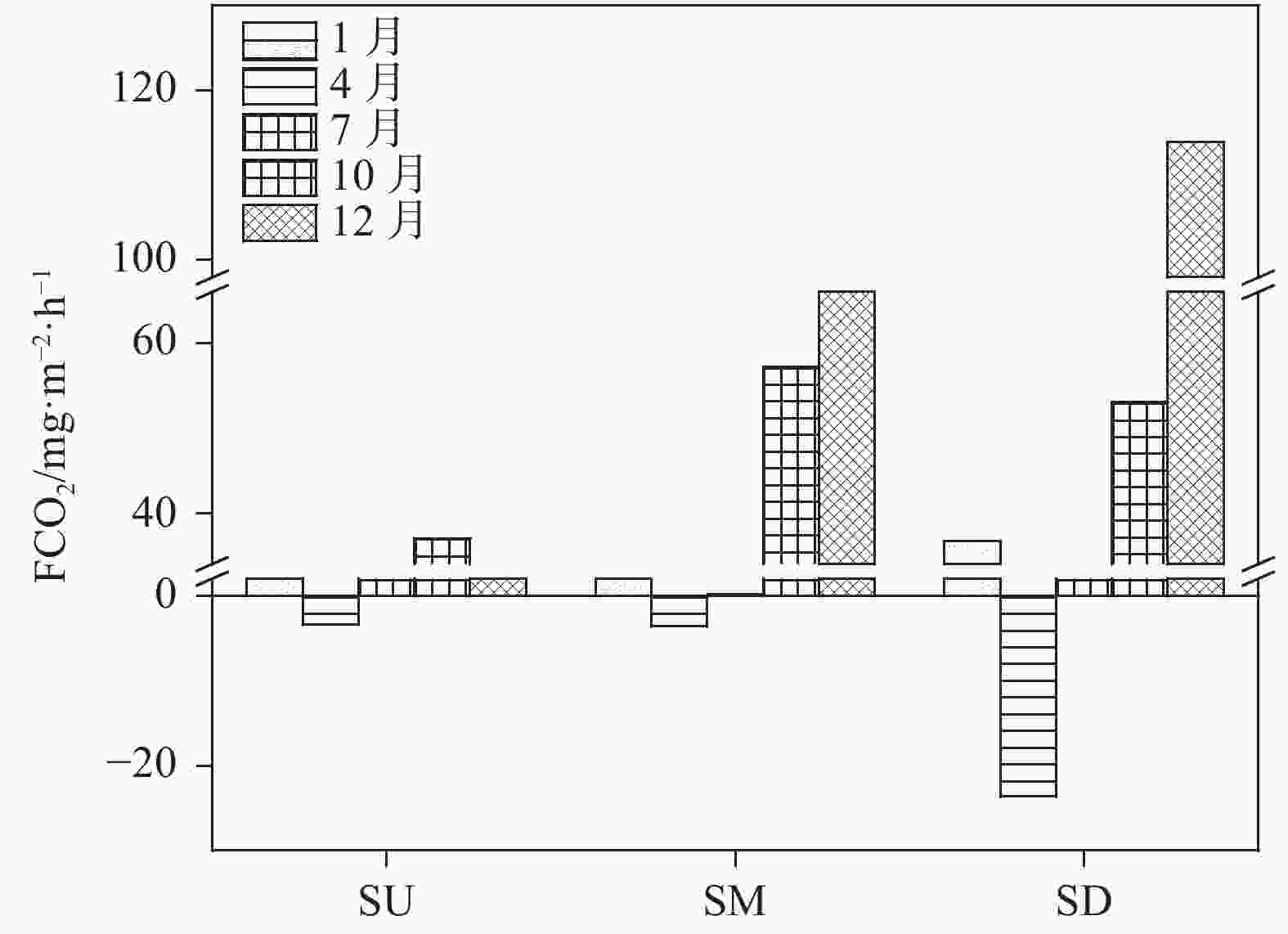
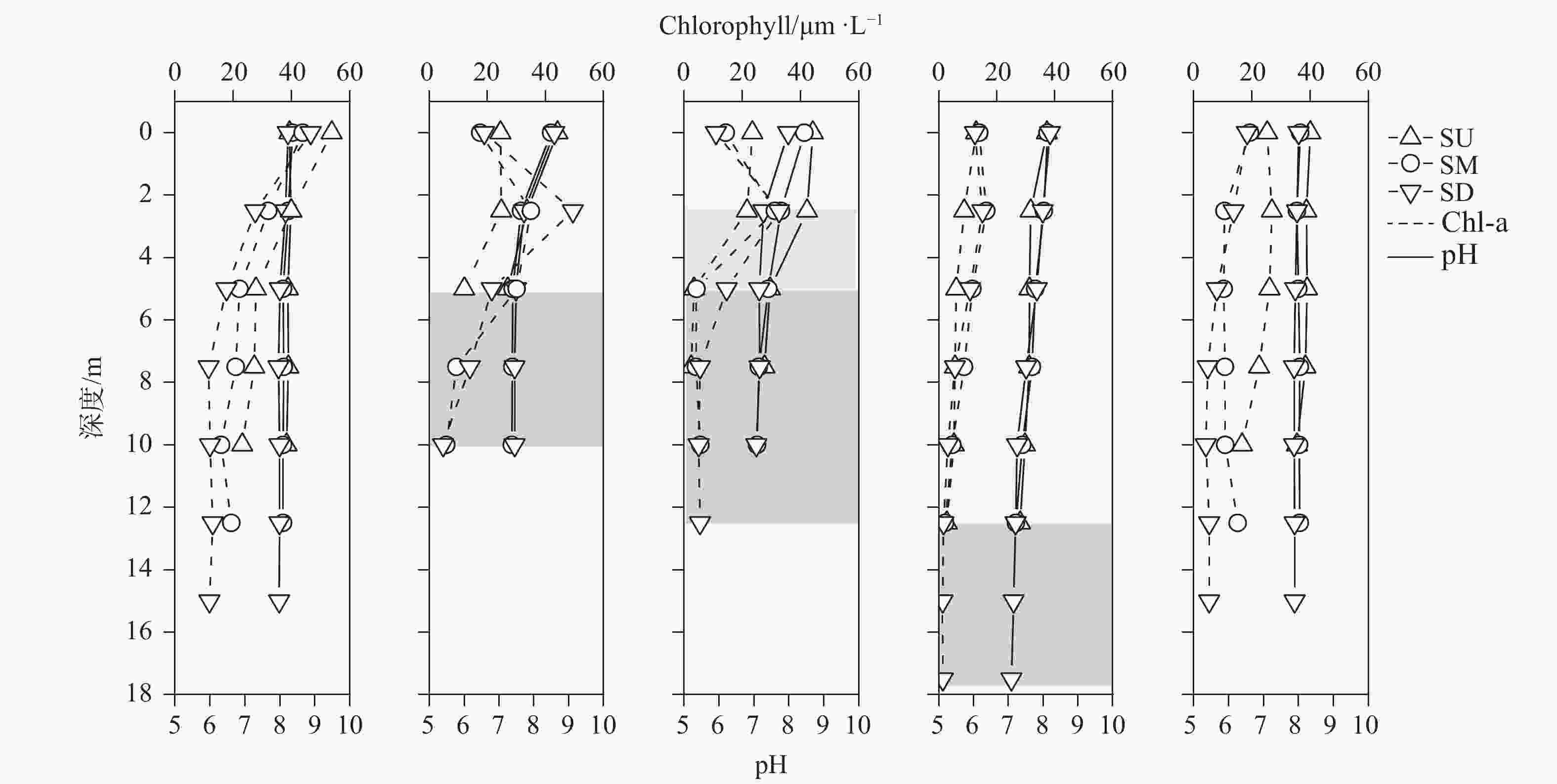
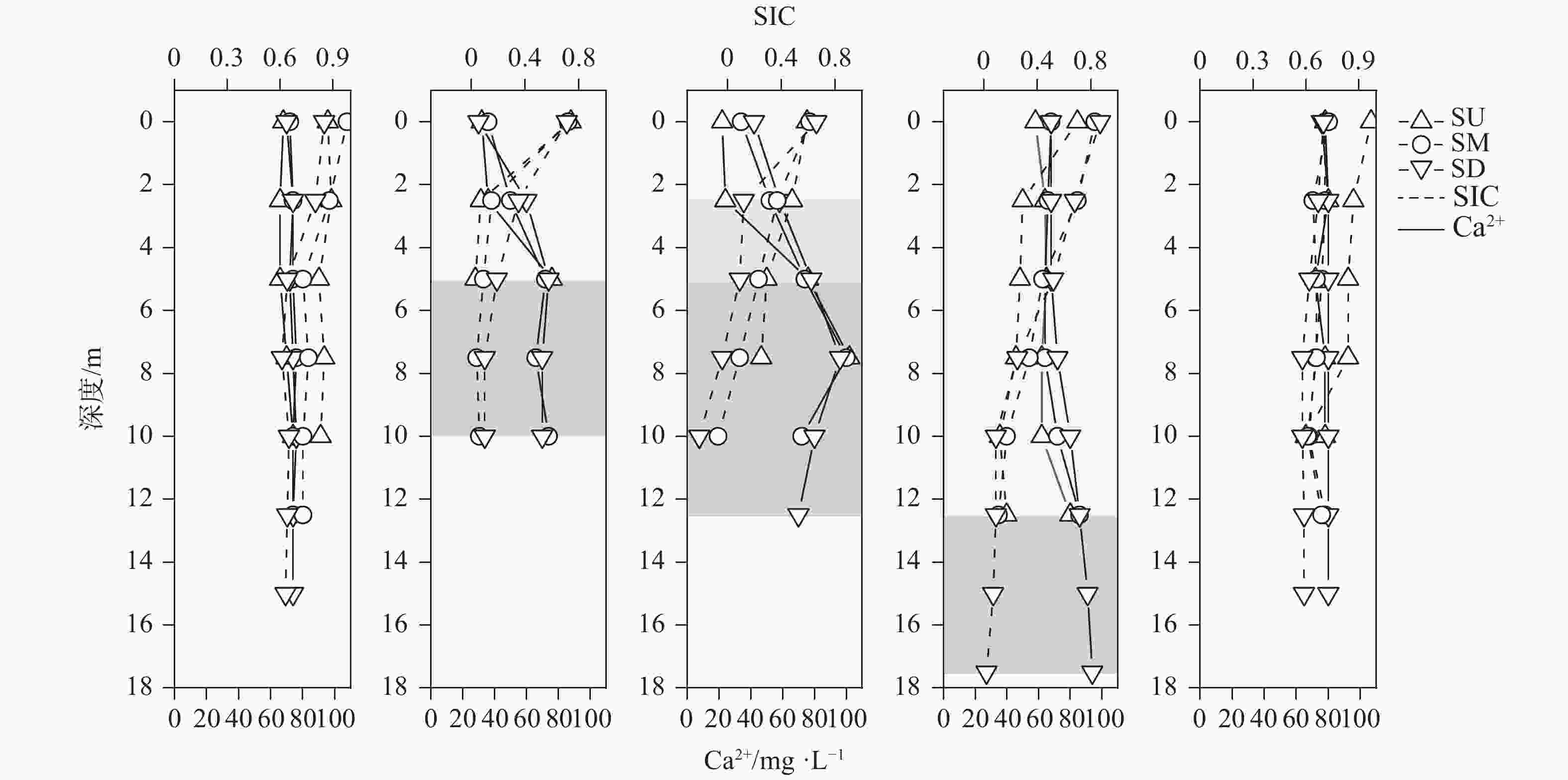
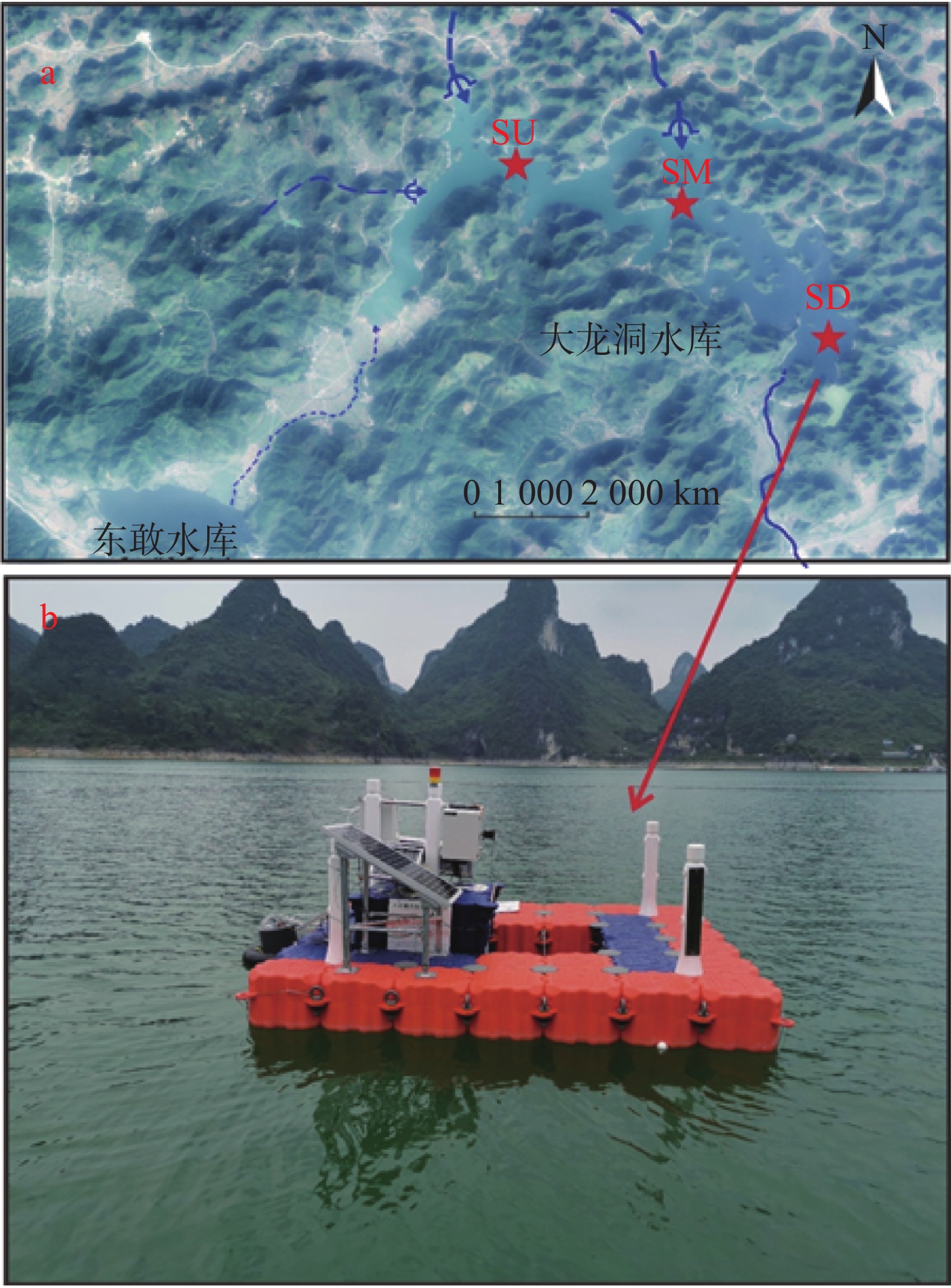
摘要: 以中国南方亚热带地区典型的地下水补给型水库——大龙洞水库为对象,于2018年1月、4月、7月、10月、12月分别在上、中、下游三个监测点进行采样,探究水库热结构变化对于水体无机碳及其同位素的影响过程及机理。结果表明:(1)大龙洞水库水体在一个水文年中呈现周期性的混合期—分层期—混合期的热结构变化,4月热分层开始显现,7月逐渐显著呈现完整的热分层,10月以后热分层逐渐消失,水体逐渐实现混合;(2)水体热分层是溶解无机碳(DIC)浓度与碳稳定同位素(δ13CDIC)值变化的主要驱动力。表水层中DIC主要受水—气界面二氧化碳脱气、水生生物光合作用控制,其DIC浓度与δ13CDIC值分别为3.22 mmol·L−1和−9.15‰;温跃层中DIC主要受有机质降解过程影响,其DIC浓度与δ13CDIC值分别为3.43 mmol·L−1和−9.70‰;底水层中DIC主要受碳酸盐沉淀过程影响,其DIC浓度与δ13CDIC值分别为4.32 mmol·L−1和−11.89‰;(3)三种过程伴随水库热结构的变化而变化,驱动DIC浓度及其同位素的变化梯度 G (DIC)与 G (δ13CDIC)的变化,表现为底水层<表水层<温跃层。热分层结束进入混合期后,DIC浓度与δ13CDIC值的时空差异均逐渐消失,最终表现出DIC浓度与δ13CDIC值的均一化。Abstract: Dalongdong Reservoir, a typical groundwater recharge reservoir in karst area of southwest China, is located at 23°30′01″−23°40′08″N and 108°30′02″−108°36′04″E in Shanglin county, Nanning City, Guangxi. This large reservoir was built mainly for irrigation and power generation by blocking caves, sinkholes and some fissures in a karst valley in 1958. It covers a rainwater collecting area of 310 km2, totaling storage capacity of 151 million m3. Approximately 95% of the recharge water comes from two karst subterranean streams. The principal strata in the reservoir catchment are carbonate rocks of Carboniferous (C1) and Devonian (D2d3) era. Influenced by the subtropical monsoon climate, the average temperature in the reservoir area is 21℃ and the average rainfall is 1,837.3 mm. The rainy season in this area is from April to September. Samples were collected respectively by the plankton method at the upper, middle and downstream monitoring sites in January, April, July, October and December, 2018. Based on DIC concentration, δ13CDIC value, water temperature, DO, SIC, partial pressure of carbon dioxide, carbon dioxide diffusion flux and other indicators, the thermal structure change on DIC concentrations and isotope composition δ13CDIC in each water layer of reservoir and its influencing factors are systematically studied. The gradient differences of DIC concentrations and isotopic composition δ13CDIC in different water layers and their causes are also discussed.In order to provide scientific and technological support for accurate assessment of carbon budget of land and water, the carbon cycle process of karst reservoirs was deeply revealed. The results show that Dalongdong reservoir presents periodic thermal structure changes in the mixing stage−stratification stage−in a hydrological year. The thermal stratification begins to appear in April, gradually presents complete thermal stratification in July, and gradually disappears after October.Besides, thermal stratification is an important driving force of DIC concentration and δ13CDIC value. After the formation of thermal stratification, DIC concentrations in the upper, middle and lower reaches show the same characteristics as those of the δ13CDIC values at the same layer, and DIC concentrations increase with depth, while δ13CDIC values become negative. DIC concentrations and δ13CDIC values are respectively 3.22 mmol·L−1 and −9.15‰ in surface water, 3.43 mmol·L−1 and −9.70‰ in thermocline, and 4.32 mmol·L−1 and −11.89‰ in bottom water. After thermal stratification, the vertical, horizontal and seasonal variations of DIC concentrations and δ13CDIC values gradually disappear, and finally show their homogenization.Finally, carbon dioxide degassing and photosynthesis dominate the water-air interface in the surface water layer. Degradation of organic matter is the main process in thermocline, and carbonate precipitation can be mostly found in the bottom water layer. The change of these three processes with the reservoir thermal structure may drive the variation of DIC concentrations and isotope gradient G (DIC) and G (δ13CDIC), which shows the characteristics of bottom water layer < surface water layer < thermocline layer.
-
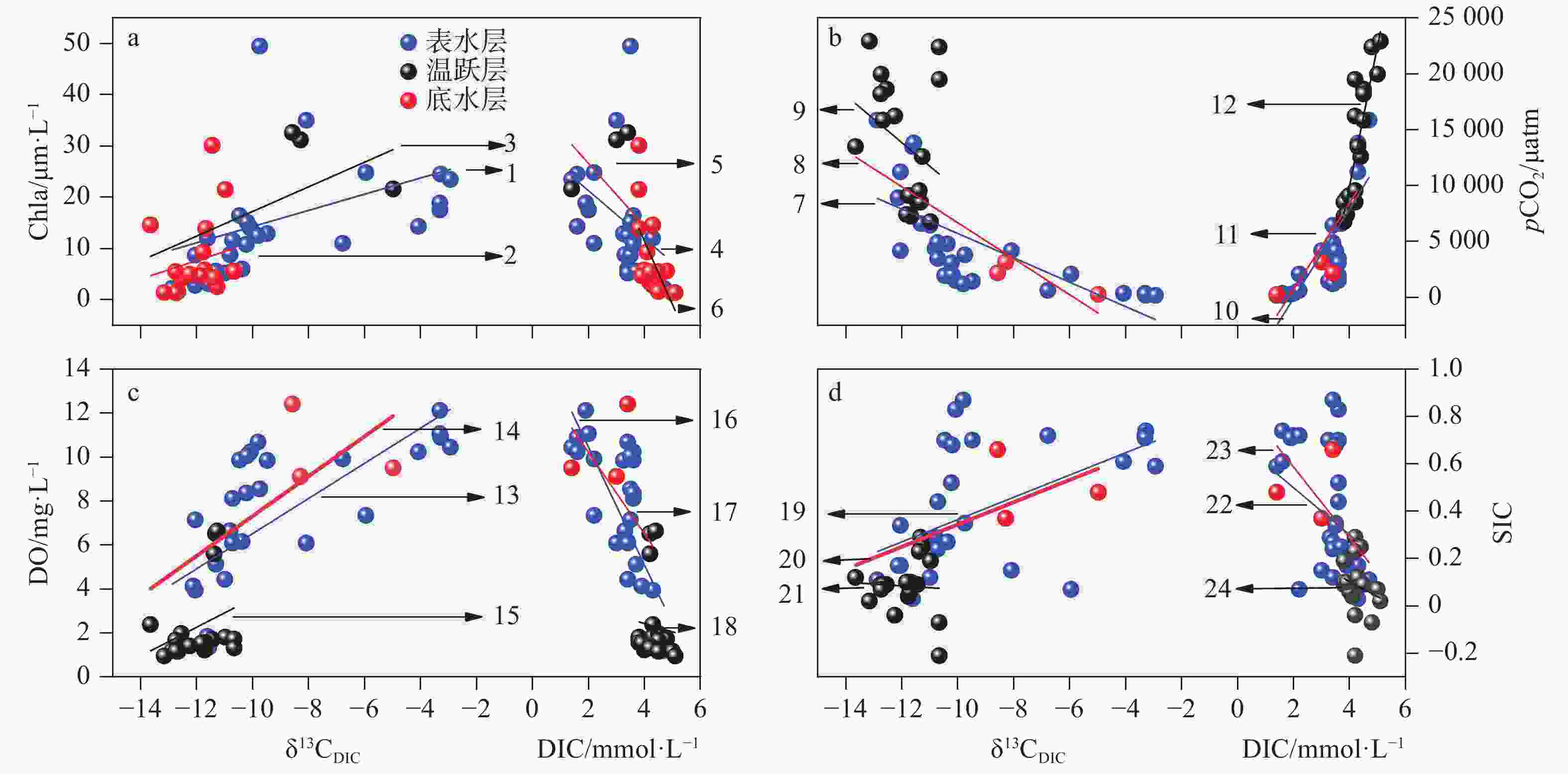
图 9 大龙洞水库各采样点分层期间各水层DIC浓度及δ13CDIC值与主要指标相关关系
Figure 9. Correlation between DIC concentrations and δ13CDIC values and the main parameters during the thermal stratification period in Dalongdong reservoir
(a:1.y=1.58x+30.07,r=0.43;2.y=2.40x+41.27,r=0.58;3.y=1.79x+29.09,r=0.20;4.y=−4.66x+30.60,r=−0.38;5.y=−6.11x+38.82,r=−0.54;6.y=−12.34x+60.77,r=−0.63; b:7.y=−1 090.13x−5 182.74,r=−0.71;8.y=−1 609.28x−9 414.79,r=−0.96;9.y=−2 106.46x−11 434.47,r=−0.32;10.y=4 009.81x−8 096.79,r=0.79;11.y=3 871.66x−7 035.17,r=0.85;12.y=12 545x−40 210.48,r=0.88; c:13.y=0.80x+14.52,r=0.70;14.y=0.91x+16.39,r=0.79;15.y=0.65x+10.11,r=0.30;16.y=−2.68x+15.81,r=−0.70;17.y=−1.85x+13.93,r=−0.59;18.y=−0.38x+3.93,r=−0.08; d:19.y=0.047x+0.84,r=0.54;20.y=0.047x+0.82,r=0.75;21.y=−0.01x−0.01,r=−0.06;22.y=−0.15x+0.88,r=−0.52;23.y=−0.01x+0.70,r=−0.58;24.y=−0.06x+0.36,r=−0.21)
表 1 监测期间各水层物理化学参数平均值
Table 1. Mean values of physical and chemical parameters of each water layer during monitoring
位置 T/ pH DIC/ δ13CDIC/ Ca2+/ DO/ Chl-a/ pCO2/ SIC ℃ mmol·L−1 ‰ mg·L−1 mg·L−1 μg·L−1 μatm
1月SU-Mix 16.64 8.25 3.64 −9.55 68.80 9.27 34.36 1072.16 0.85 SM-Mix 16.77 8.16 3.75 −10.35 74.00 8.23 25.66 1339.14 0.80 SD-Mix 17.09 8.04 3.83 −11.29 73.43 7.12 20.02 1763.48 0.69
4月SU-E 23.47 7.91 2.70 −6.95 48.00 6.70 20.43 5255.04 0.28 SM-E 22.70 7.84 2.93 −7.61 52.67 6.29 27.53 4347.72 0.32 SM-H 17.60 7.38 3.97 −11.64 70.67 1.43 15.07 8917.20 0.06 SD-E 22.23 7.93 3.07 −8.01 54.67 7.49 29.97 3614.85 0.42 SD-H 17.23 7.46 3.83 −11.49 71.33 1.63 13.37 7139.43 0.13
7月SU-E 29.00 8.60 1.40 −3.96 23.00 9.98 22.52 215.41 0.54 SU-T 26.70 7.99 2.80 −8.15 50.00 8.02 12.50 4384.21 0.39 SU-H 24.15 7.38 4.30 −11.30 89.00 6.58 2.95 10550.32 0.27 SM-E 28.75 8.11 2.30 −6.18 43.00 9.68 22.75 1758.55 0.49 SM-T 26.20 7.59 3.60 −9.83 63.00 7.36 17.75 6356.10 0.30 SM-H 23.23 7.21 4.50 −11.53 82.00 3.10 4.67 16852.67 0.08 SD-E 28.75 8.16 2.80 −7.68 50.00 11.17 21.80 1409.36 0.69 SD-T 26.05 7.63 3.85 −11.12 68.00 7.40 23.60 7838.70 0.39 SD-H 21.73 7.16 4.30 −12.33 81.00 1.69 7.65 16850.80 −0.01
10月SU-E 22.82 7.63 3.51 −10.74 65.17 6.19 6.88 5528.57 0.30 SM-E 22.88 7.70 3.82 −11.39 70.00 6.78 9.60 5976.82 0.43 SD-E 22.97 7.66 3.83 −10.96 73.67 6.12 8.12 7099.00 0.42 SD-H 21.33 7.15 4.87 −12.85 90.33 1.09 1.43 19570.08 0.06
12月SU-Mix
SM-Mix
SD-Mix17.18
17.38
17.638.20
8.02
7.923.55
3.59
3.76−10.21
−10.73
−11.4177.20
75.00
79.439.05
7.47
6.5223.50
12.90
8.601178.11
1791.03
2441.430.82
0.67
0.62注:SU(上游), SM(中游), SD(下游), E(表水层), T(温跃层), H(底水层),Mix(混合期)。 表 2 观测期间大龙洞水库各水层DIC浓度与δ13CDIC值变化梯度
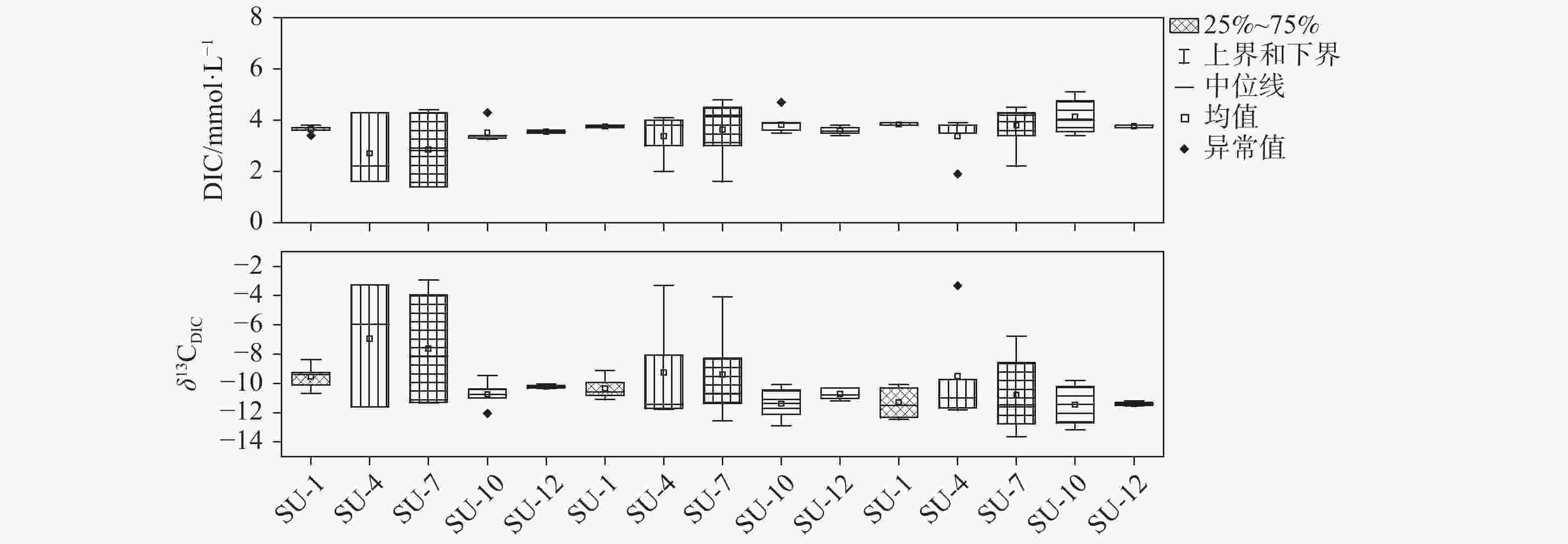
Table 2. Gradient differences of DIC concentrations and δ13CDIC values at each water depth during study period (G (DIC), G (δ13CDIC)
1月 4月 7月 10月 12月
混合期
表水层SU
SM
SD
SU(0.04,0.23)
(0.01,0.14)
(0.01,0.16)
(0.54,1.67)
(0,0.82)
(0.08,0.21)(0.01,0.03)
(0.03,0.07)
(0.01,0.02)SM (0.36,1.63) (0.56,1.68) (0.10,0.22) SD (0.38,1.53) (0.48,0.72) (0.09,0.23)
温跃层SU (1.11,2.54) SM (0.48,1.23) SD (0.36,2.03)
底水层SU (0.08,0.02) SM (0.04,0.06) (0.12,0.38) SD (0.02,0.17) (0.04,0.40) (0.12,0.01) -
[1] Barros N, Cole J J, Tranvik L J, Prairie Y T, Bastviken D, Huszar V. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude[J]. Nature Geoscience, 2011, 4(9):593-596. doi: 10.1038/ngeo1211 [2] Dean W E, Gorham E. Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands[J]. Geology, 1998, 26(6):535-538. doi: 10.1130/0091-7613(1998)026<0535:MASOCB>2.3.CO;2 [3] Tranvik L J, Downing J A, Cotner J B, Loiselle S A, Striegl R G, Ballatore T J, Dillon P, Finlay K, Fortino K, Knoll L B. Lakes and reservoirs as regulators of carbon cycling and climate[J]. Limnology and Oceanography, 2009, 54(6):2298-2314. [4] Cole J J, Prairie Y T, Caraco N F, Mcdowell W H, Tranvik L J, Striegl R G, Duarte C M, Kortelainen P, Downing J A, Middelburg J J, Melack J. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget[J]. Ecosystems, 2007, 10(1):172-185. doi: 10.1007/s10021-006-9013-8 [5] Aufdenkampe A K, Mayorga E, Raymond P A, Melack J M, Doney S C, Alin S R, Aalto R E, Yoo K. Riverine coupling of biogeochemical cycles between land,oceans,and atmosphere[J]. Frontiers in Ecology and the Environment, 2011, 9(1):53-60. doi: 10.1890/100014 [6] Dynesium M, Nilsson C. Fragmentation and flow regulation of river systems in the northern third of the world[J]. Science, 1994, 266(5186):753-762. doi: 10.1126/science.266.5186.753 [7] Jaramillo F, Destouni G. Local flow regulation and irrigation raise global human water consumption and footprint[J]. Science, 2015, 350(6265):1248-1251. doi: 10.1126/science.aad1010 [8] Lehner B, Liermann C R, Revenga C, Smarty V R, Fekete B C, Crouzet P, Li P D, Endejan M, Frenken K, Magome J. High-resolution mapping of the world's reservoirs and dams for sustainable river-flow management[J]. Frontiers in Ecology and the Environment, 2011, 9(9):494-502. doi: 10.1890/100125 [9] Teodoru C R, DelGiorgio P A, Prairie Y T, Annick St-Pierre. Depositional fluxes and sources of particulate carbon and nitrogen in natural lakes and a young boreal reservoir in Northern Québec[J]. Biogeochemistry, 2013, 113(1-3):323-339. doi: 10.1007/s10533-012-9760-x [10] Wen Z D, Song K S, Shang Y X, Fang C, Li L, Lv L L, Lv X G, Chen L J. Carbon dioxide emissions from lakes and reservoirs of China: a regional estimate based on the calculated pCO2[J]. Atmospheric Environment, 2017, 170:71-81. [11] 刘丛强, 汪福顺, 王雨春, 王宝利. 河流筑坝拦截的水环境响应: 来自地球化学的视角[J]. 长江流域资源与环境, 2009, 18(4):384-396. doi: 10.3969/j.issn.1004-8227.2009.04.015LIU Congqiang, WANG Fushu, WANG Yuchun, WANG Baoli. Responses of aquatic environment to river damming—from the geoche mical view[J]. Resources and Environment in the Yangtze Basin, 2009, 18(4):384-396. doi: 10.3969/j.issn.1004-8227.2009.04.015 [12] Anderson L G, Jutterström S, Hjalmarsson S, Wåhlström I, Semiletov I P. Outgassing of CO2 from Siberian Shelf seas by terrestrial organic matter decomposition[J]. Geophysical Research Letters, 2009, 36:390-406. [13] van Geldern R, Schulte P, Mader M, Baier A, Barth J A. Spatial and temporal variations of pCO2, dissolved inorganic carbon and stable isotopes along a temperate karstic watercourse[J]. Hydrological Process, 2015, 29(1515):3423-3440. [14] Baehr M M, DeGrandpre M D. In situ pCO2 and O2 measurements in a lake during turnover and stratification[J]. Limnology and oceanography, 2004, 49(2):330-340. doi: 10.4319/lo.2004.49.2.0330 [15] Aberg J, Jansson M, Jonsson A. Importance of water temperature and thermal stratification dynamics for temporal variation of surface water CO2 in a boreal lake[J]. Journal of Geophysical^ Research, 2010, 115(G02024). [16] Wang W, Li S L, Zhong J, Li C, Yi Y, Chen S, Ren Y. Understanding transport and transformation of dissolved inorganic carbon (DIC) in the reservoir system using δ13CDIC and water chemistry[J]. Journal of Hydrology, 2019, 574:193-201. [17] Khadka M B, Martin J B, Jin J. Transport of dissolved carbon and CO2 degassing from a river system in a mixed silicate and carbonate catchment[J]. Journal of Hydrology, 2014, 513:391-402. [18] Doctor D H, Kendall C, Sebestyen S D, Shanley J B, Ohte N, Boyer E W. Carbon isotope fractionation of dissolved inorganic carbon (DIC) due to outgassing of carbon dioxide from a headwater stream[J]. Hydrological Process, 2008, 22(14):2410-2423. doi: 10.1002/hyp.6833 [19] 赵登忠, 谭德宝, 李翀, 申邵洪. 隔河岩水库二氧化碳通量时空变化及影响因素[J]. 环境科学, 2017, 38(3):954-963.ZHAO Dengzhong, TAN Debao, LI Chong, SHEN Shaohong. Tempo-spatial variations and influential factors of carbon dioxide emissions from the geheyan reservoir over the qingjiang river basin, China[J]. Environmental Science, 2017, 38(3):954-963. [20] 刘文, 蒲俊兵, 于奭, 章程, 区绎如, 袁道先, 杨会, 唐伟. 广西五里峡水库夏季溶解无机碳行为的初步研究[J]. 环境科学, 2014, 35(8):2959-2966.LIU Wen, PU Junbing, YU Shi, ZHANG Cheng, QU Yiru, YUAN Daoxian, YANG Hui, TANG Wei. Preliminary research on the feature of dissolved inorganic carbon in wulixia reservoir in summer, Guangxi, China[J]. Environmental Science, 2014, 35(8):2959-2966. [21] Miao Liu, Yunlin Zhang, Kun Shi. Thermal stratification dynamics in a large and deep subtropical reservoir revealed by high-frequency buoy data[J]. Science of the Total Environment, 2019, 651:614-624. doi: 10.1016/j.scitotenv.2018.09.215 [22] Downing J A, Duarte C M. The global abundance and size distribution of lakes, ponds, and impoundments[J]. Limnology and oceanography, 2006, 51(5):2388-2397. doi: 10.4319/lo.2006.51.5.2388 [23] 黄思宇. 典型岩溶地下水补给型水库碳埋藏机制研究[D]. 北京: 中国地质科学院, 2020: 1-125.HUANG Siyu. Carbon Burial in a Typical Groundwater-fed Reservoir in Karst area[D]. Beijing: Chinese Academy of Geological Science, 2020: 1-125. [24] 吴飞红, 蒲俊兵, 李建鸿, 张陶, 李丽, 黄思宇. 夏季热分层效应对典型岩溶水库水化学及溶解无机碳的影响[J]. 环境科学, 2017, 38(8):3210-3217.WU Feihong, PU Junbing, LI Jianhong, ZHANG Tao, LI Li, HUANG Siyu. Impacts of thermal stratification on the hydrochemistry and dissolved inorganic carbon in a typical karst reservoir in summer[J]. Environmental Science, 2017, 38(8):3210-3217. [25] 李建鸿, 蒲俊兵, 袁道先, 刘文, 肖琼, 于奭, 张陶, 莫雪, 孙平安, 潘谋成. 岩溶区地下水补给型水库表层无机碳时空变化特征及影响因素[J]. 环境科学, 2015, 36(8):2833-2842.LI Jianhong, PU Junbing, YUAN Daoxian, LIU Wen, XIAO Qiong, YU Shi, ZHANG Tao, MO Xue, SUN Pingan, PAN Moucheng. Variations of inorganic carbon and its impact factors in surface-layer waters in a groundwater-fed reservoir in karst area, SW China[J]. Environmental Science, 2015, 36(8):2833-2842. [26] Pu J, Li J, Zhang T, Martin J B, Yuan D. Varying thermal structure controls the dynamics of CO2 emissions from a subtropical reservoir, south China[J]. Water Research, 2020, 178:115831. doi: 10.1016/j.watres.2020.115831 [27] Huang S H, Pu J B, Cao J H, Li J, Bai B. Origin and effect factors of sedimentary organic carbon in a karst groundwater-fed reservoir, south china[J]. Environmental Science and Pollution Research, 2018, 25(8):8497-8511. [28] 张陶, 李建鸿, 蒲俊兵, 吴飞红, 李丽, 袁道先. 桂江流域夏季水气界面CO2脱气的空间变化及其影响因素[J]. 环境科学, 2017, 38(7):2773-2783.ZHANG Tao, LI Jianhong, PU Junbing, WU Feihong, LI Li, YUAN Daoxian. Spatial variations of CO2 degassing across water-air interface and its impact factors in summer in Guijiang river, China[J]. Environmental Science, 2017, 38(7):2773-2783. [29] Kelly C A, Rudd J W M, Bodaly R A, Roulet N P, Stlouis V L, Heyes A, Moore T R, Schiff S, Aravena R, Scott K J. Increases in fluxes of greenhouse gases and methyl mercury following flooding of an experimental reservoir[J]. Environmental Science and Technology, 1997, 31(5):1334-1344. doi: 10.1021/es9604931 [30] Jacob K. 古滨河. 湖沼学: 内陆水生态系统[M]. 北京: 高等教育出版社, 2011: 154-180.Jacob K. Gu Binhe. Limnology: Inland aquatic ecosystems[M]. Beijing: Higher Education Press, 2011: 154-180. [31] Khairul Hasan, Kaosar Alam, Md Saidul Azam Chowdhury. The Use of an Aeration System to Prevent Thermal Stratification of Water Bodies: Pond, Lake and Water Supply Reservoir[J]. Applied Ecology and Environmental Sciences, 2014, 2(1):1-7. [32] Lewis W M Jr. A revised classification of lakes based on mixing[J]. Canadian Journal of Fisheries and Aquatic Sciences, 1983, 40(10):1779-1787. doi: 10.1139/f83-207 [33] Das A, Krishnaswami S, Bhattacharya S K. Carbon isotope ratio of dissolved inorganic carbon (DIC) in rivers draining the Deccan Traps, India: Sources of DIC and their magnitudes[J]. Earth and planetary science Letters, 2005, 236(1-2):419-429. doi: 10.1016/j.jpgl.2005.05.009 [34] Khadka M B, Martin J B, Jin J. Transport of dissolved carbon and CO2 degassing from a river system in a mixed silicate and carbonate catchment[J]. Journal of Hydrology, 2014, 513:391-402. doi: 10.1016/j.jhydrol.2014.03.070 [35] Schindler D W. Recent advances in the understanding and management of eutrophication[J]. Limnology and Oceanography, 2006, 51(1, part 2):356-363. [36] Reynolds C S, Irish A E, Elliott J A. The ecological basis for simulating phytoplankton responses to environmental change (PROTECH)[J]. Ecological Modelling, 2001, 140(3):271-291. doi: 10.1016/S0304-3800(01)00330-1 [37] Gamier J, Billen G, Coste M. Seasonal succession of diatoms and Chlorophyceae in the drainage network of the Seine River: observation and modeling[J]. Limnology and Oceanography, 1995, 40(4):750-765. doi: 10.4319/lo.1995.40.4.0750 [38] Yao G R, Gao Q Z, Wang Z G, Huang X, Tong Y, Zhang Y, Jiao S, Jian D. Dynamics of CO2 partial pressure and CO2 outgassing in the lower reaches of the Xijiang River, a subtropical monsoon river in China[J]. Science of the Total Environment, 2007, 376(1-3):255-266. doi: 10.1016/j.scitotenv.2007.01.080 [39] Guo J H, Wang F S, Vogt R D, Zhang Y, Liu C Q. Anthropogenically enhanced chemical weathering and carbon evasion in the Yangtze Basin[J]. Scientific Reports, 2015, 5(1):11941. doi: 10.1038/srep11941 [40] Wang X D, Yang S Y, Ran X B, Liu X M, Bataille, Clement. Response of the Changjiang (Yangtze River) water chemistry to the impoundment of Three Gorges Dam during 2010–2011[J]. Chemical Geology, 2018, 487:1-11. [41] Bardhan P, Naqvi S W A, Karapurkar S G, Shenoy D M, Kurian S, Naik H. Isotopic composition of nitrate and particulate organic matter in a pristine dam reservoir of western India: implications for biogeochemical processes[J]. Biogeosciences, 2017, 14(4):767-779. doi: 10.5194/bg-14-767-2017 [42] Maberly S C, Barker P A, Stott A W, Ville M D. Catchment productivity controls CO2 emissions from lakes[J]. Nature Climate Change, 2012, 3(4):391-394. [43] Raymond P A, Hartmann J, Lauerwald R, Sobek S, Guth P. Global carbon dioxide emissions from inland waters[J]. Nature, 2013, 503(7476):355-359. doi: 10.1038/nature12760 [44] De V M, Martin J B, Cohen M J, Foster C, Kurz M J. Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river[J]. Chemical Geology, 2011, 283(1-2):31-43. [45] Hartley A M, House W A, Leadbeater B S, Callow M E. The use of microelec trodes to study the precipitation of calcite upon algal biofilms[J]. Journal of Colloid and Interface Science, 1996, 183(2):498-505. doi: 10.1006/jcis.1996.0573 -




 下载:
下载: