Simulation of buffering process and carbon sink effect of lime soil on sulfuric acid rain
-
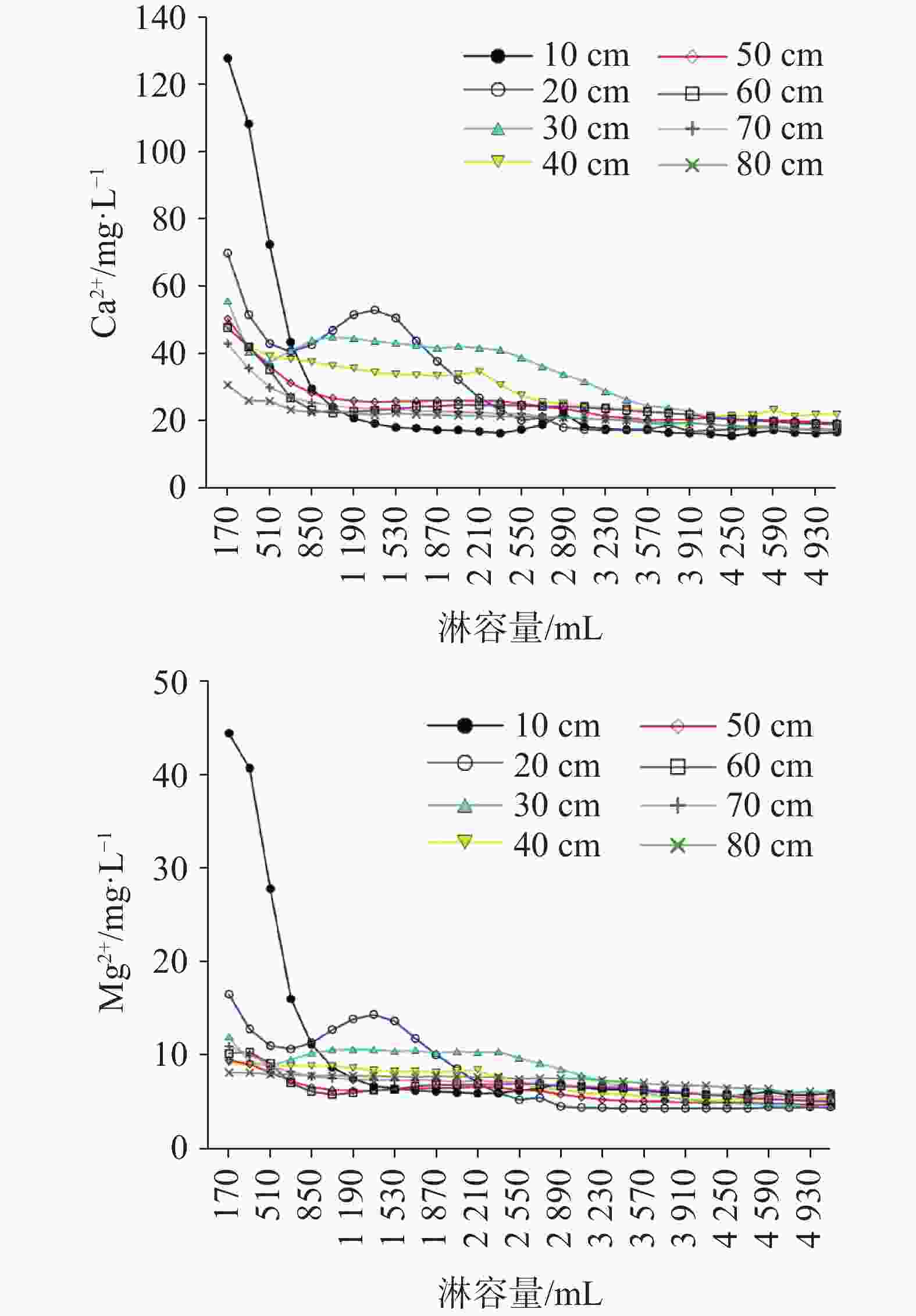
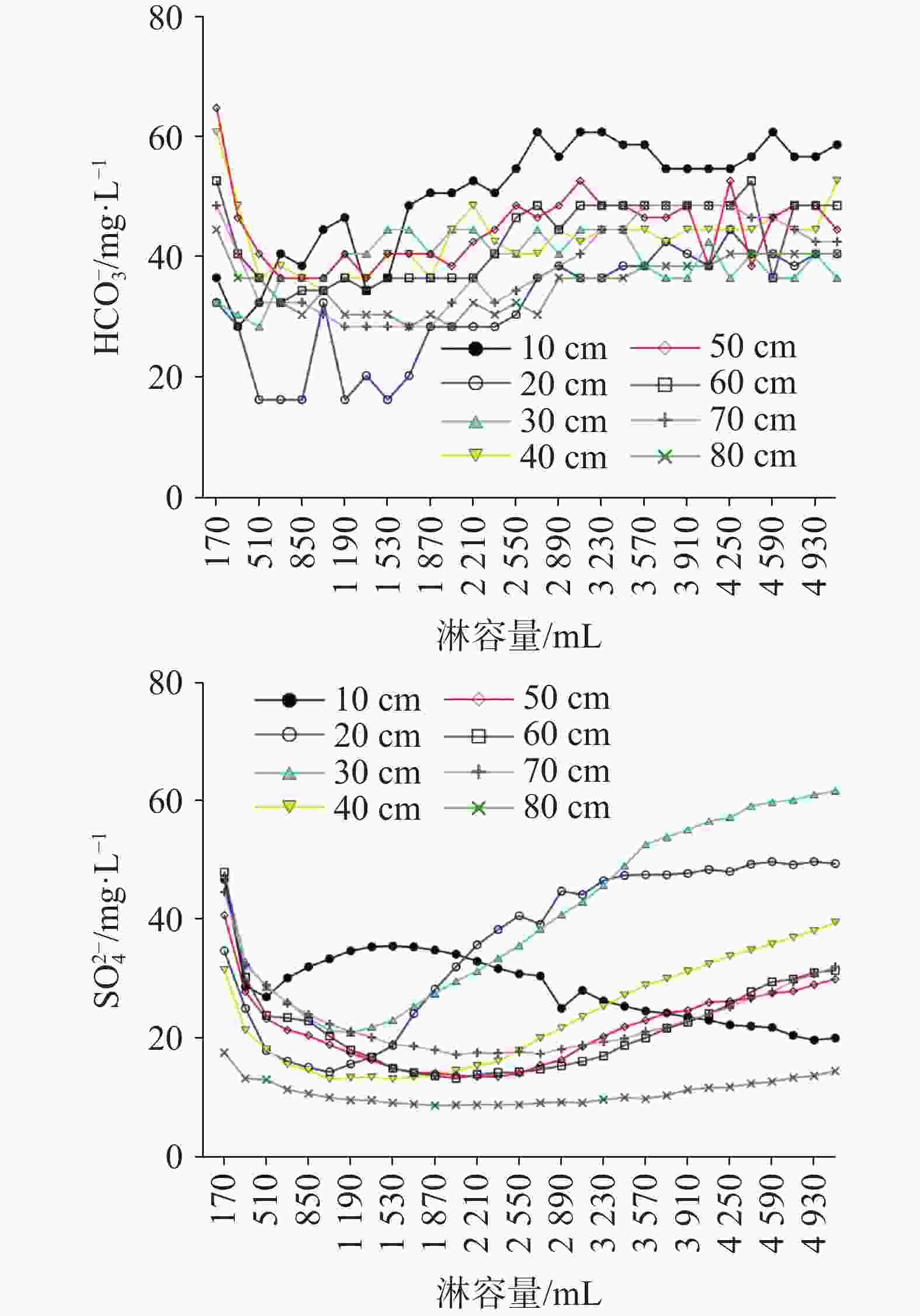
摘要: 硫酸型酸雨沉降至地表经石灰土缓冲后,参与碳酸盐岩溶蚀及对岩溶碳汇的影响尚不明确,严重制约了我国岩溶碳汇效应的准确评估。本研究通过设置不同土层厚度条件下pH 4.5的硫酸型酸雨淋滤实验,以明确石灰土对硫酸型酸雨的缓冲过程及关键控制因素。结果表明:石灰土对酸雨的缓冲作用主要发生在表层(10 cm),淋出液中Ca2+、Mg2+、
${\rm{HCO}}_3^{-}$ 含量在淋溶初期均表现快速降低,当淋溶量(土壤水达饱和后)为1 020 mL时Ca2+、Mg2+、${\rm{HCO}}_{{3}}^{-}$ 淋失量趋于稳定,稳定淋失量分别为20 mg·L−1、6 mg·L−1、40 mg·L−1。淋出液中被酸雨H+交换出的Ca2+、Mg2+仅占很小一部分,土壤水溶性Ca2+、Mg2+是淋出液中Ca2+、Mg2+的主要部分,开放系统中,大气和土壤CO2溶于降雨形成H2CO3不仅增加碳汇,且H2CO3解离产生的H+交换土壤中交换态Ca2+、Mg2+,造成Ca2+、Mg2+的淋失量不容忽视。不同厚度石灰土中交换性钙镁可缓冲酸雨容量均大于土壤碳酸钙矿物可缓冲容量,前者是后者的1.17~1.59倍。相同酸度、同一降雨量(土壤水达饱和后)下土壤盐基离子参与酸雨缓冲产生的碳汇量约为碳酸盐矿物风化缓冲产生碳汇量的2.1倍,不同厚度(≥10 cm)石灰土产生的碳汇量大致相等。根据本次实验及桂林市降雨数据计算,桂林市质纯石灰岩风化残积土壤区(土层厚度≥10 cm),土壤盐基离子参与酸雨缓冲每年可产生0.59 ~0.93 mol·m−2的碳汇通量。Abstract: After sulfuric acid rain settles to the surface and is buffered by lime soil, its participation in carbonate rock erosion and its impact on karst carbon sink are still unclear, which seriously restricts the accurate assessment of karst carbon sink effect in China. In order to clarify the buffering process and key control factors of lime soil to sulfuric acid rain, we conducted leaching experiments of sulfuric acid rain with pH=4.5 under different soil thicknesses. Results show that contents of Ca2+, Mg2+ and${\rm{HCO}}_3^{-}$ in leaching solution decrease rapidly at the initial stage of leaching. When the leaching amount (after soil water reaches saturation) reaches 1,020 mL, the leaching loss of Ca2+, Mg2+ and${\rm{HCO}}_3^{-}$ tends to be stable, and stable leaching amounts are 20 mg·L−1, 6 mg·L−1 and 40 mg·L−1 respectively. The same ion in leaching solution of different thicknesses of soil columns shows the same trend, which indicates that the lime soil buffering of acid rain may mainly occur in the 10 cm (surface) soil layer. Ca2+ and Mg2+ exchanged by acid rain H+ in leaching solution only accounts for a small part, and the soil water-soluble Ca2+ and Mg2+ is the main part of Ca2+ and Mg2+ in leaching solution. In an open system, atmospheric and soil CO2 dissolves in rainfall to form H2CO3, which not only increases carbon sink, but also exchanges Ca2+ and Mg2+ in soil with H+ generated by the dissociation of H2CO3. The leaching amount of Ca2+ and Mg2+as a result cannot be ignored. The special physical structure of topsoil may produce preferential flow, which will significantly reduce the exchangeable point of H+ in leachate, causing dissolution of carbonate minerals to assist the buffering of H+ in leachate. The karst area is the main place for carbon loss in terrestrial ecosystems, and the most intense carbon leaching occurs in surface soil. Exchangeable calcium, exchangeable magnesium and carbonate minerals in lime soil are principal reactants for buffering acid rain. The buffering capacity of exchangeable calcium and magnesium in lime soil with different thicknesses is greater than that of soil calcium carbonate minerals, and the former is 1.17-1.59 times as large as that of the latter. Under the same acidity and the same rainfall (after the soil water reaches saturation), the carbon sink generated by soil base ions participating in acid rain buffering is about 2.1 times as large as that generated by weathering buffering of carbonate minerals. Under the same rainfall conditions, the existence of lime soil can significantly increase carbon sink, and the carbon sink generated by lime soil with different thicknesses (≥10 cm) is roughly equal. According to this experiment and the rainfall data of Guilin, in the weathered residual soil area of pure limestone in Guilin (soil thickness greater than 10 cm), the participation of soil base ions in acid rain buffering can produce 0.59-0.93 mol·m−2 carbon sink flux per year.-
Key words:
- lime soil /
- sulfuric acid rain /
- base ions /
- buffer mechanism /
- carbon sink flux
-
表 1 供试土壤基本理化性质
Table 1. Physico-chemical properties of soil samples
石灰土厚度/cm 10 20 30 40 50 60 70 80 pH 6.62 6.66 6.51 6.63 6.62 6.52 6.54 6.92 容重/g·cm−3 1.47 1.37 1.24 1.33 1.58 1.68 1.63 1.62 含水率/% 23.2 26.6 24.7 27.9 29.7 27.0 25.6 27.4 水溶性Ca2+/×10-6 5.75 11.70 8.97 10.90 8.89 3.10 11.80 8.02 交换性钙/cmol·kg−1 7.4 4.7 4.2 4.6 6.4 4.8 5.0 5.3 水溶性Mg2+/×10-9 3.48 3.89 4.77 6.42 5.90 4.69 5.90 6.02 交换性镁/cmol·kg−1 2.4 1.9 1.7 2.1 2.4 2.5 2.7 2.9 碳酸钙/×10-3 6.83 8.34 2.77 3.00 3.70 5.55 7.30 5.09 表 2 石灰土淋出液水化学分析结果 (mg·L−1)
Table 2. Hydrochemical analysis results of leachate from lime soil (mg·L−1)
土层厚度/cm pH Ca2+ Mg2+ K+ Na+ ${\rm{HCO}}_3^{-}$ ${\rm{SO}}_4^{2-}$ ${\rm{NO}}_3^{-}$ Cl− 10
(n=30)最大值 7.71 127.79 44.44 8.06 6.90 60.63 33.35 547.59 13.94 最小值 7.15 15.68 5.72 2.97 0.59 28.29 19.83 3.99 1.48 平均值 7.52 27.68 10.00 3.69 1.30 50.26 24.31 61.33 3.18 标准差 0.17 27.07 9.87 1.33 1.32 9.52 3.11 138.60 3.18 变异系数 2.30 97.79 98.61 36.18 101.77 18.94 12.79 225.98 100.30 20
(n=30)最大值 7.46 69.97 16.61 12.51 5.36 44.46 34.83 249.84 14.56 最小值 6.42 17.08 4.40 6.21 0.77 16.17 17.14 4.86 1.90 平均值 7.00 30.20 7.84 8.48 1.61 31.60 28.01 81.15 4.36 标准差 0.28 15.34 4.02 2.27 0.97 9.25 6.61 86.15 2.77 变异系数 4.00 50.79 51.32 26.83 60.20 29.26 23.61 106.15 63.56 30
(n=30)最大值 7.53 55.79 12.04 6.77 5.05 44.46 40.76 175.98 10.50 最小值 6.73 19.04 4.64 4.10 0.92 28.29 20.44 4.77 1.90 平均值 7.18 33.79 8.19 5.42 1.79 39.28 30.34 77.23 4.62 标准差 0.25 10.54 2.47 0.94 0.86 4.40 7.17 54.16 1.86 变异系数 3.51 31.18 30.16 17.30 48.09 11.20 23.63 70.13 40.40 40
(n=30)最大值 7.57 48.20 9.30 4.82 5.24 60.63 29.72 119.70 7.74 最小值 6.88 21.55 5.29 3.36 1.26 34.36 16.52 13.02 3.11 平均值 7.23 29.60 7.10 3.99 2.01 42.98 21.73 59.98 4.37 标准差 0.14 7.49 1.53 0.54 0.92 5.38 4.51 41.55 1.04 变异系数 1.95 25.32 21.54 13.43 45.68 12.53 20.77 69.28 23.75 50
(n=30)最大值 7.60 50.54 9.54 4.37 6.03 64.67 30.32 115.02 8.77 最小值 6.82 19.55 4.74 2.81 1.16 36.38 16.77 5.72 3.10 平均值 7.23 25.58 6.14 3.25 2.08 44.53 20.69 41.80 4.14 标准差 0.22 6.91 1.22 0.41 1.08 6.24 3.24 31.25 1.43 变异系数 3.10 26.99 19.89 12.70 51.97 14.02 15.67 74.76 34.54 60
(n=30)最大值 7.50 47.84 10.43 3.72 7.26 52.55 33.96 125.59 9.41 最小值 6.85 19.24 5.16 2.28 1.38 32.34 16.60 3.52 3.60 平均值 7.16 24.84 6.67 2.74 2.23 42.84 20.78 39.90 4.63 标准差 0.18 6.28 1.27 0.36 1.20 6.59 3.87 32.11 1.48 变异系数 2.45 25.29 19.10 12.97 53.78 15.39 18.64 80.48 32.06 70
(n=30)最大值 7.45 43.18 11.03 2.62 5.87 48.50 32.28 109.33 9.60 最小值 6.89 17.63 5.56 1.63 1.39 28.29 18.60 3.30 3.60 平均值 7.21 22.93 7.11 1.92 2.19 38.74 21.66 40.08 4.92 标准差 0.15 5.43 1.27 0.22 0.95 7.60 3.10 29.48 1.42 变异系数 2.02 23.68 17.87 11.46 43.28 19.62 14.32 73.56 28.88 80
(n=30)最大值 7.38 30.82 8.25 1.08 3.38 44.46 18.78 62.97 8.53 最小值 6.74 17.10 6.04 0.75 1.60 28.29 14.33 16.36 4.88 平均值 7.08 21.31 7.39 0.89 2.02 35.30 15.41 45.09 5.81 标准差 0.19 2.89 0.66 0.07 0.38 4.49 1.08 15.33 0.87 变异系数 2.64 13.54 8.90 8.33 18.84 12.73 7.01 33.99 15.03 注:n为样品数量。 表 3 不同厚度土壤Ca2+、Mg2+含量及可参与淋溶量
Table 3. Contents of Ca2+ and Mg2+ in soil with different thicknesses and the amount that can be involved in leaching
石灰土厚度/cm 10 20 30 40 50 60 70 80 单层水溶性Ca2+/mg 21.48 39.78 28.03 35.61 34.04 12.84 48.12 32.04 累积水溶性Ca2+/mg 21.48 61.26 89.29 124.90 158.94 171.78 219.90 251.94 水溶性Ca2+可参与淋溶量/L 1.07 3.06 4.46 6.24 7.95 8.59 11.00 12.60 单层水溶性Mg2+/mg 13.00 13.23 14.91 20.97 22.59 19.43 24.06 24.05 累积水溶性Mg2+/mg 13.00 26.22 41.13 62.10 84.70 104.13 128.19 152.24 水溶性Mg2+可参与淋溶量/L 2.17 4.37 6.86 10.35 14.12 17.36 21.37 25.37 表 4 不同厚度土壤累积Ca2+、Mg2+含量及淋失比
Table 4. Accumulative content of Ca2+ and Mg2+ in soil with different thicknesses and its leaching-loss ratio
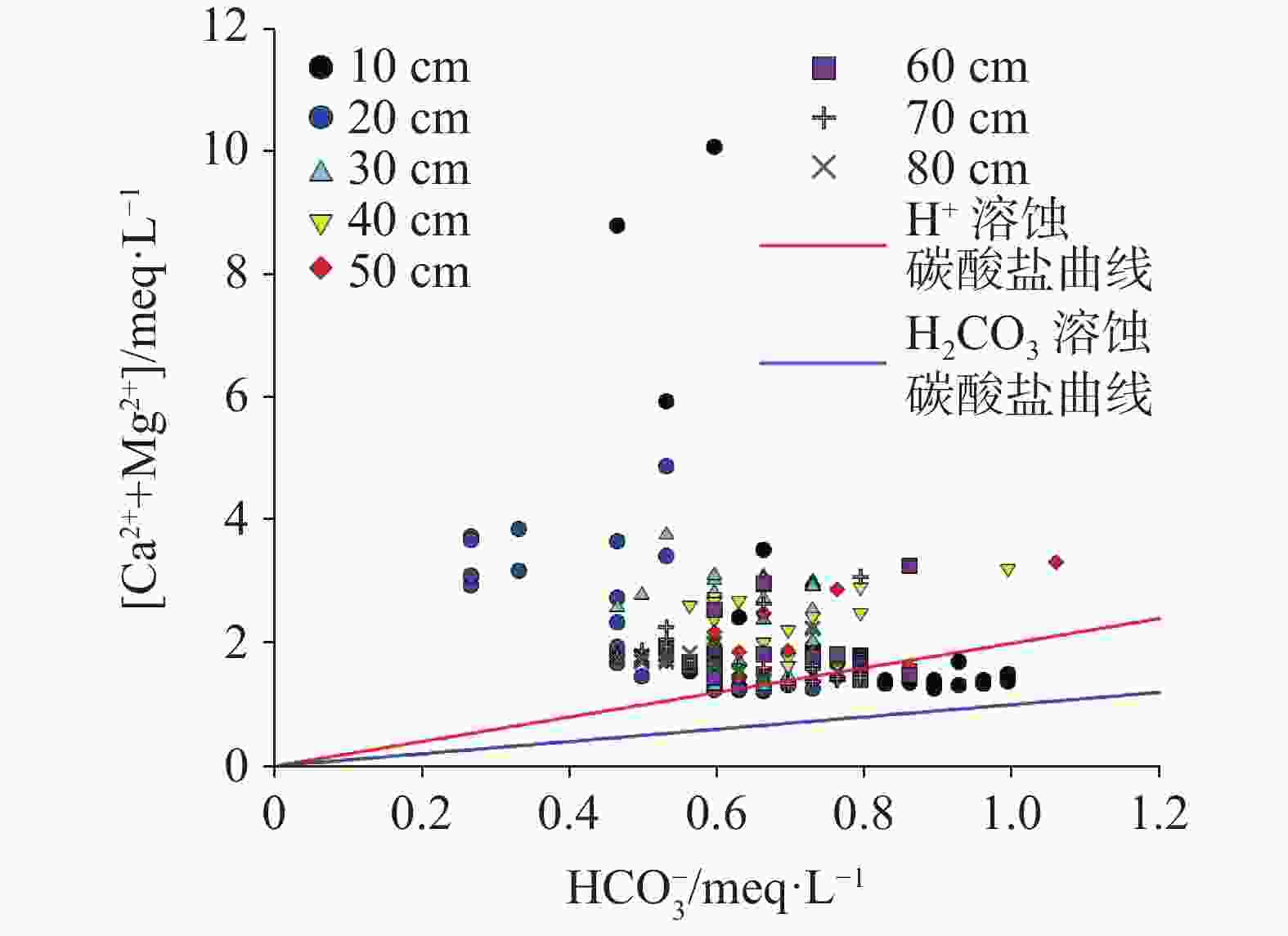
石灰土厚度/cm 10 20 30 40 50 60 70 80 累积土壤水溶性Ca2+/meq 1.07 3.06 4.46 6.24 7.95 8.59 11.00 12.60 累积土壤水溶性Mg2+/meq 1.08 2.19 3.43 5.18 7.06 8.68 10.68 12.69 累积土壤水溶性Ca2++Mg2+/meq 2.16 5.25 7.89 11.42 15.00 17.27 21.68 25.28 淋出液水溶性Ca2++Mg2+/meq 6.95 8.23 8.65 6.81 5.25 5.43 5.47 5.46 水溶性Ca2++Mg2+淋失比/% 321.76 156.76 109.63 59.63 35.00 31.44 25.23 21.60 累积土壤交换性Ca2+/mg 11056 17448 22698 28709 38511 46466 54622 63091 累积土壤交换性Ca2+/meq 553 872 1135 1435 1926 2323 2731 3155 累积土壤交换性Mg2+/mg 2151 3702 4977 6623 8829 11315 13957 16738 累积土壤交换性Mg2+/meq 179 308 415 552 736 943 1163 1395 累积土壤交换性Ca2++Mg2+/meq 732 1180 1550 1987 2662 3266 3894 4550 淋出液交换性Ca2++Mg2+/meq 4.36 2.80 3.44 3.75 3.88 3.74 3.40 3.11 交换性Ca2++Mg2+淋失比/% 0.60 0.24 0.22 0.19 0.15 0.11 0.09 0.07 表 5 不同厚度石灰土可缓冲酸雨(pH=4.5)容量
Table 5. Capacity of buffer acid rain(pH=4.5) in different thicknesses of lime
石灰土厚度/cm 10 20 30 40 50 60 70 80 土壤交换性Ca2++Mg2+/meq 732 1 180 1 550 1 987 2 662 3 266 3 894 4 550 淋出液交换性Ca2++Mg2+/meq 4.36 2.80 3.44 3.75 3.88 3.74 3.40 3.11 H交换态/mm(pH=4.5) 27 269 68 449 73 184 86 061 111 434 141 836 186 019 237 625 土壤碳酸钙以钙计/g 10.20 21.55 25.01 28.93 34.60 43.79 55.70 63.84 溶蚀矿物态钙/g 0.09 0.06 0.07 0.08 0.08 0.07 0.07 0.06 H矿物态/mm(pH=4.5) 18408 58 336 58 030 58 735 70 247 101 606 129 240 172 815 H交换态/ H矿物态 1.48 1.17 1.26 1.47 1.59 1.40 1.44 1.38 表 6 酸雨沉降下不同厚度石灰土产生的碳汇通量
Table 6. The carbon sink flux of lime soil with different thicknesses caused by acid rain deposition
石灰土厚度/cm 10 20 30 40 50 60 70 80 碳汇量by/mmol 4.20 2.64 3.28 3.59 3.72 3.58 3.24 2.95 碳汇量bt/mmol 2.02 1.24 1.56 1.72 1.78 1.71 1.54 1.40 碳汇量by/碳汇量bt 2.08 2.13 2.10 2.09 2.09 2.09 2.10 2.11 碳汇量CY/mol·m−2·y−1 0.93 0.59 0.73 0.80 0.83 0.79 0.72 0.65 碳汇量CT/mol·m−2·y−1 0.45 0.28 0.35 0.38 0.39 0.38 0.34 0.31 -
[1] Han G L, Liu C Q. Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province, China[J]. Chemical Geology, 2004, 204(1-2):1-21. doi: 10.1016/j.chemgeo.2003.09.009 [2] Ding H, Lang Y C, Liu C Q, et al. Chemical characteristics and δ34S-SO42− of acid rain: Anthropogenic sulfate deposition and its impacts on CO2 consumption in the rural karst area of southwest China[J]. Geochemical Journal, 2013, 47(6):625-638. doi: 10.2343/geochemj.2.0293 [3] Huang Q B, Qin X Q, Liu P Y, et al. Impact of sulfuric and nitric acids on carbonate dissolution, and the sociated deficit of CO2 uptake in the upper-middle reaches of the Wujiang River, China[J]. Journal of Contaminant Hydrology, 2017, 203:18-27. doi: 10.1016/j.jconhyd.2017.05.006 [4] 于奭. 西江流域河流化学风化及其碳汇效应研究[D]. 武汉: 中国地质大学, 2019.YU Shi. Study on chemical weathering and carbon sink in Xijiang basin[D]. Wuhan: China University of Geosciences, 2019. [5] 刘丛强, 蒋颖魁, 陶发祥, 郎赟超, 李思亮. 西南喀斯特流域碳酸盐岩的硫酸侵蚀与碳循环[J]. 地球化学, 2008, 37(4):404-414. doi: 10.3321/j.issn:0379-1726.2008.04.014LIU Congqiang, JIANG Yingkui, TAO Faxiang, LANG Yunchao, LI Siliang. Chemical weathering of carbonate rocks by sulfuric acid and the carbon cycling in Southwest China[J]. Geochimica, 2008, 37(4):404-414. doi: 10.3321/j.issn:0379-1726.2008.04.014 [6] 黄奇波, 覃小群, 程瑞瑞, 李腾芳, 刘朋雨. 硫酸型酸雨参与碳酸盐岩溶蚀的研究进展[J]. 中国岩溶, 2019, 38(2):149-156.HUANG Qibo, QIN Xiaoqun, CHENG Ruirui, LI Tengfang, LIU Pengyu. Research progress of sulfuric acid rain participating in the dissolution of carbonate rocks[J]. Carsologica Sinica, 2019, 38(2):149-156. [7] 袁道先, 蔡桂鸿. 岩溶环境学[M]. 重庆: 重庆出版社, 1988: 24-27YUAN Daoxian, CAI Guihong. The science of karst environment[M]. Chongqing: Chongqing Publishing House, 1988: 24-27 [8] Matschullat J, Andreae H, Lessmann D, et al. Catchment acidification-from the top down[J]. Environmental Pollution, 1992, 77(2-3):143-150. doi: 10.1016/0269-7491(92)90070-Q [9] 黄芬, 肖琼, 尹伟璐, 胡刚. 岩溶系统中土壤氮肥施用对岩溶碳汇的影响[J]. 中国岩溶, 2014, 33(4):405-411. doi: 10.11932/karst20140403HUANG Fen, XIAO Qiong, YIN Weilu, HU Gang. The effects of using N-fertilizers in soil on karst carbon sink in karst system[J]. Carsologica Sinica, 2014, 33(4):405-411. doi: 10.11932/karst20140403 [10] Hartikainen H. Soil response to acid percolation: Acid-base buffering and cation leaching[J]. Journal of Environmental Quality, 1996, 25(4):638-645. [11] 廖柏寒, 蒋青. 酸沉降与我国南方森林土壤的酸化[J]. 农业环境保护, 2002, 21(2):110-114.LIAO Baihan, JIANG Qing. Acid deposition and acidification of forest soils in southern China[J]. Agro-environmental Protection, 2002, 21(2):110-114. [12] 岑慧贤, 王树功, 仇荣亮, 马灵芳. 模拟酸雨对土壤盐基离子的淋溶释放影响[J]. 环境污染与防治, 2001, 23(1):13-16. doi: 10.3969/j.issn.1001-3865.2001.01.005CEN Huixian, WANG Shugong, QIU Rongliang, MA Lingfang. Effect of simulating acid rain on cation release of some soils[J]. Environmental Pollution & Control, 2001, 23(1):13-16. doi: 10.3969/j.issn.1001-3865.2001.01.005 [13] 杨忠芳, 余涛, 唐金荣, 朱翠娟, 宗思锋, 张娇, 张建新, 申志军. 湖南洞庭湖地区土壤酸化特征及机理研究[J]. 地学前缘, 2006, 13(1):105-112.YANG Zhongfang, YU Tao, TANG Jinrong, ZHU Cuijuan, ZONG Sifeng, ZHANG Jiao, ZHANG Jianxin, SHEN Zhijun. A study of the characteristics and mechanisms of soil acidification in the Dongting Lake region in Hunan Province, South China[J]. Earth Science Frontiers, 2006, 13(1):105-112. [14] 耿增超, 贾宏涛. 土壤学[M]. 第二版. 北京: 科学出版社, 2020.GENG Zengchao, JIA Hongtao. Soil Science[M]. Second Edition. Beijing: Science Press, 2020. [15] 廖柏寒, 李长生. 土壤对酸沉降缓冲机制探讨[J]. 环境科学, 1989, 10(1):30-34. doi: 10.3321/j.issn:0250-3301.1989.01.001LIAO Baihan, LI Changsheng. Study on buffering mechanism of acid precipitation in soil[J]. Environmental Science, 1989, 10(1):30-34. doi: 10.3321/j.issn:0250-3301.1989.01.001 [16] Liu K H, Mansell R S, Rhue R D. Cation removal during application of acid solutions into air-dry soil columns[J]. Soil Science Society of America Journal, 1990, 54(6):1747-1753. doi: 10.2136/sssaj1990.03615995005400060040x [17] 凌大炯, 章家恩, 黄倩春, 韩维栋, 欧阳颖. 模拟酸雨对砖红壤盐基离子迁移和释放的影响[J]. 土壤学报, 2007, 44(3):444-450. doi: 10.3321/j.issn:0564-3929.2007.03.010LING Dajiong, ZHANG Jiaen, HUANG Qianchun, HAN Weidong, OU Yangying. Influences of simulated acid rain on leaching and release of base ions in latosol[J]. Acta Pedologica Sinica, 2007, 44(3):444-450. doi: 10.3321/j.issn:0564-3929.2007.03.010 [18] 于俊霞, 焦燕, 杨文柱, 刘立家, 宋春妮, 于亚泽. 外源盐对盐碱土壤无机碳淋溶特征的影响[J]. 环境科学学报, 2021, 41(6):2358-2368. doi: 10.13671/j.hjkxxb.2020.0500YU Junxia, JIAO Yan, YANG Wenzhu, LIU Lijia, SONG Chunni, YU Yaze. Influence of exogenous salt on inorganic carbon leaching in saline-alkali soil[J]. Acta Scientiae Circumstantiae, 2021, 41(6):2358-2368. doi: 10.13671/j.hjkxxb.2020.0500 [19] 李春龙, 赵家梅, 龙偲, 陈中吉, 周运超, 张春来. 模拟酸雨条件下石灰土-碳酸盐岩体系的碳汇效应[J]. 中国岩溶, 2014, 33(1):51-56. doi: 10.3969/j.issn.1001-4810.2014.01.008LI Chunlong, ZHAO Jiamei, LONG Si, CHEN Zhongji, ZHOU Yunchao, ZHANG Chunlai. Carbon sink effect of simulated acid rain in lime soil and carbonate rock system[J]. Carsologica Sinica, 2014, 33(1):51-56. doi: 10.3969/j.issn.1001-4810.2014.01.008 [20] 刘炜, 周运超, 张春来. 石灰土盐基离子迁移对模拟酸雨的响应[J]. 中国岩溶, 2018, 37(3):336-342.LIU Wei, ZHOU Yunchao, ZHANG Chunlai. Response of base cations migration of lime soil to simulated acid rain[J]. Carsologica Sinica, 2018, 37(3):336-342. [21] 吴春笃, 戴竞, 阿琼, 陈诗龙, 解清杰. 酸雨的研究现状及新兴趋式的可视化分析[J]. 安全与环境学报, 2019, 19(1):344-353. doi: 10.13637/j.issn.1009-6094.2019.01.051WU Chundu, DAI Jing, A Qiong, CHEN Shilong, XIE Qingjie. Visualization analysis of the research status and emerging trends of the acid rain application[J]. Journal of Safety and Environment, 2019, 19(1):344-353. doi: 10.13637/j.issn.1009-6094.2019.01.051 [22] Pan Y, Birdsey R A, Fang J, et al. A large and persistent carbon sink in the world’s forests[J]. Science, 2011, 333(6045):988-993. doi: 10.1126/science.1201609 [23] 解淑艳, 王胜杰, 于洋, 刘丽, 张凤英. 2003-2008年全国酸雨状况变化趋势研究[J]. 中国环境监测, 2020, 36(4):80-88.XIE Shuyan, WANG Shengjie, YU Yang, LIU Li, ZHANG Fengying. Analysis on acid rain status and its change trends in China from 2003 to 2008[J]. Environmental Monitoring in China, 2020, 36(4):80-88. [24] 李陵, 黎泳珊, 彭良, 周艺, 柴发合, 莫招育, 陈志明, 李红. 桂林市酸雨污染特征及防治对策研究[J]. 环境科学研究, 2020, 33(6):1393-1401. doi: 10.13198/j.issn.1001-6929.2020.03.11LI Ling, LI Yongshan, PENG Liang, ZHOU Yi, CHAI Fahe, MO Zhaoyu, CHEN Zhiming, LI Hong. Pollution characteristics and control countermeasures of acid rain in Guilin[J]. Research of Environmental Sciences, 2020, 33(6):1393-1401. doi: 10.13198/j.issn.1001-6929.2020.03.11 [25] 陈怀满. 环境土壤学[M]. 第三版. 北京: 科学出版社, 2018.CHEN Huaiman. Environmental soil science[M]. Third Edition. Beijing: Science Press, 2018. [26] 俞元春, 丁爱芳, 胡笳, 孟磊. 模拟酸雨对土壤酸化和盐基迁移的影响[J]. 南京林业大学学报, 2001, 25(2):39-42.YU Yuanchun, DING Aifang, HU Jia, MENG Lei. Effects of simulated acid rain on soil acidification and base ions transplant[J]. Journal of Nanjing Forestry University, 2001, 25(2):39-42. [27] 黄奇波, 吴华英, 程瑞瑞, 李腾芳, 罗飞, 赵光帅, 李小盼. 桂林岩溶区石灰土壤对酸雨缓冲作用的观测及其对岩溶碳汇的指示意义[J/OL]. 地球学报, https://kns.cnki.net/kcms/detail/11.3474.p.20220121.1039.002.html.HUANG Qibo, WU Huaying, CHENG Ruirui, LI Tengfang, LUO Fei, ZHAO Guangshuai, LI Xiaopan. Buffering effect of lime soil on acid rain and its influence on the evaluation of the karst carbon sink effect[J/OL]. Acta Geoscientica Sinica, https://kns.cnki.net/kcms/detail/11.3474.p.20220121.1039.002.html. [28] Schulze E D, Luyssaert S, Ciais P, et al. Importance of methane and nitrous oxide for Europe’s terrestrial greenhouse-gas balance[J]. Nature Geoscience, 2009, 2(12):842-850. doi: 10.1038/ngeo686 [29] 廖柏寒, 戴昭华. 土壤对酸沉降的缓冲能力与土壤矿物的风化特征[J]. 环境科学学报, 1991, 11(4):425-431. doi: 10.13671/j.hjkxxb.1991.04.007LIAO Baihan, DAI Zhaohua. Soil buffering capacity to acid precipitation and weathering characteristics of soil minerals[J]. Acta Scientiae Circumstantiae, 1991, 11(4):425-431. doi: 10.13671/j.hjkxxb.1991.04.007 [30] 章程, 汪进良, 肖琼, 郭永丽, 苗迎. 桂林潮田河水溶解无机碳昼夜变化与通量[J]. 地球学报, 2021, 42(4):555-564. doi: 10.3975/cagsb.2020.110301ZHANG Cheng, WANG Jinliang, XIAO Qiong, GUO Yongli, MIAO Ying. Day and night variations of dissolved inorganic carbon and flux in Chaotian River, Guilin, Guangxi[J]. Acta Geoscientica Sinica, 2021, 42(4):555-564. doi: 10.3975/cagsb.2020.110301 [31] 肖佩文, 肖保华. 土柱淋洗实验研究进展及其在土壤有机碳迁移研究中的应用展望[J]. 地球与环境, 2021, 49(1):106-114. doi: 10.14050/j.cnki.1672-9250.2020.48.096XIAO Peiwen, XIAO Baohua. Research progress of soil column leaching experiment and its application prospect in soil organic carbon migration[J]. Earth and Environment, 2021, 49(1):106-114. doi: 10.14050/j.cnki.1672-9250.2020.48.096 [32] Mcfee W W, Kelly J M, Beck R H. Acid precipitation effects on soil pH and base saturation of exchange sites[J]. Water, Air and Soil Pollution, 1977, 7(3):401-408. -








 下载:
下载: