Analysis of influencing factors on mineral morphology of active speleothemA case study of Furong cave in Chongqing
-
摘要: 石笋是古气候重建的重要地质载体,文石与方解石是石笋碳酸钙晶体的常见矿物形态。根据现代洞穴监测数据分析洞穴新生碳酸钙沉积物 (Active Speleothem: AS)的矿物形态的研究较少。本文在重庆武隆芙蓉洞三个滴水点 (MP2、MP5、MP9)下放置玻璃片,收集新生碳酸钙沉积物和滴水样品,监测新生碳酸钙沉积物矿物形态、滴水的Mg/Ca比值、pH、滴率和洞穴环境等指标,分析玻璃片正面和反面新生碳酸钙沉积物的δ18O、δ13C和Mg/Ca比值。研究发现:(1) MP2滴水点下的玻璃片正反面新生碳酸钙沉积物的矿物形态均为方解石;MP5和MP9滴水点的正面沉积方解石和文石-方解石混合两种情况,反面沉积文石-方解石,且反面文石多于正面。 (2) MP2滴水Mg/Ca比值小于MP5和MP9,说明滴水Mg/Ca比值是影响新生碳酸钙沉积物矿物形态的重要因素;而滴水pH值对AS矿物形态的影响在不同滴水点有差异。(3) 不论是玻璃片正面还是反面,文石-方解石混合的新生碳酸钙沉积物δ18O和δ13C比以方解石为主的沉积物偏正,说明AS矿物形态的变化会导致δ18O和δ13C发生变化。通过在芙蓉洞的系统监测和分析,发现新生碳酸钙沉积物的矿物形态与地表环境、洞穴上部岩溶水文地质条件密切相关,并验证了洞穴新生碳酸钙沉积物的矿物形态对石笋δ18O和δ13C具有重要影响。Abstract: Stalagmites, secondary mineral deposits forming in karst caves, record much paleo-climate and paleo-environment information. In stalagmites, the mineral forms of calcium carbonate are aragonite and calcite. It is considered that the properties of bedrock, discharge of drip water, pH, and the Mg/Ca ratios of drip water are the important factors affecting the crystal morphology. In addition, the changes of mineral morphology in stalagmites are thought to indicate the changes of paleo-climate and paleo-environment. At present, most studies focus on inferring the change of paleoclimate through the crystal morphology in stalagmites, while there are few studies on analyzing the mineral crystal morphology of active speleothem (AS) according to modern cave monitoring data. In this study, in order to collect active speleothem and drip water samples during 2017-2018, glass plates were placed under 3 drip water sites (MP2, MP5, MP9) in Furong Cave located in Wulong District, Chongqing. The mineral crystal morphology of AS was identified by polarizing microscope. Systematic monitoring was performed on Mg/Ca ratios, pH, the discharge of drip water, the cave environment, as well as δ18O, δ13C and Mg/Ca ratios of the active speleothem deposited on the front and back sides of glass plates. The results suggest that, (1) The mineral crystal morphology of active speleothem that deposit on both sides of glass plates at MP2 is calcite. There are calcite and aragonite-calcite mixture on the front side of glass plates at MP5 and MP9. However, aragonite-calcite crystals deposit on the back side of the glass plates, and there are more aragonites than those on the front sides. (2) The Mg/Ca ratios of drip water collected from MP2 is less than the ratios from MP5 and MP9, which indicates that the Mg/Ca ratio of drip water is an important factor affecting the mineral morphology of active speleothem. And the effect of pH values of drip water on AS mineral crystal morphology is different at different drip sites. (3) Regardless of the front or back side of glass plates, the δ18O and δ13C of AS mixed with aragonite-calcite are more positive than the calcite-dominated AS, which suggests that changes in the AS mineral morphology will lead to changes in δ18O and δ13C. The systematic monitoring and analysis in Furong Cave show that the mineral form of AS is closely related to the surface environment and the karst hydrogeological conditions in the upper part of the cave, and it has been verified that the mineral form of AS in caves has important influence on the δ18O and δ13C of stalagmites.
-
Key words:
- drip water /
- active speleothem /
- calcite /
- aragonite /
- Mg/Ca
-
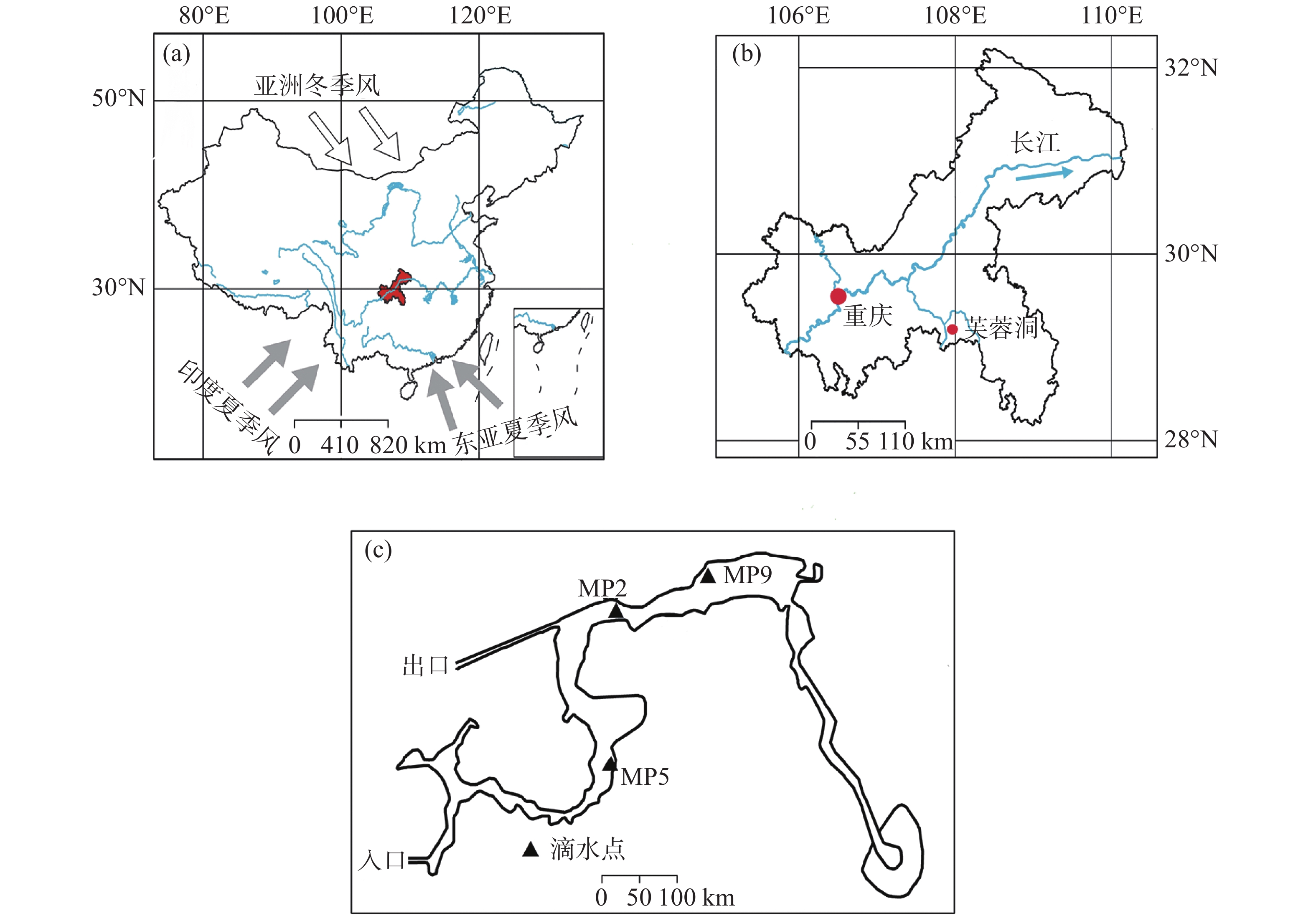
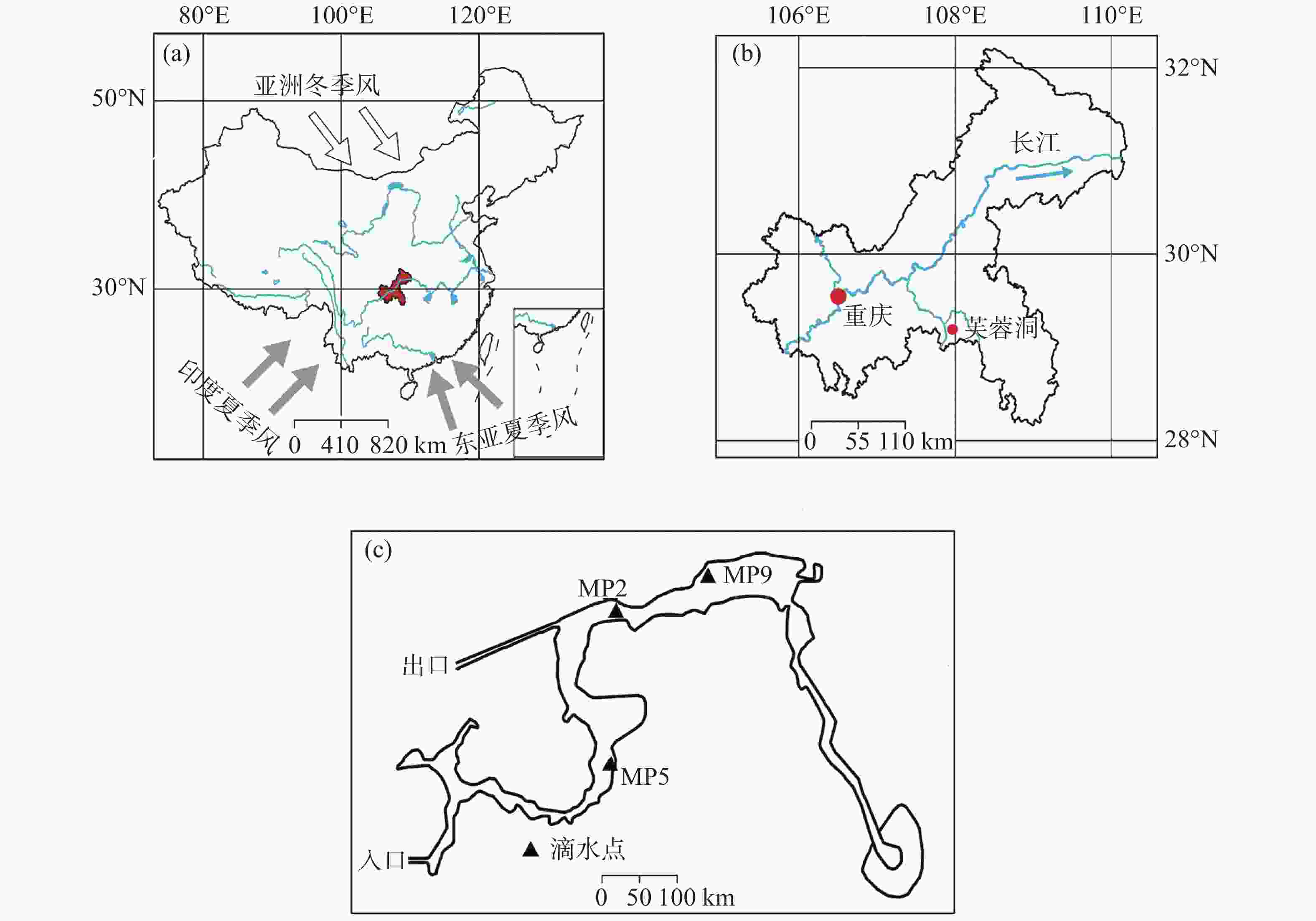
图 1 (A) 研究区的位置。灰色箭头为印度夏季风和东亚夏季风,白色箭头为亚洲冬季风),红色区域为重庆市所在位置;(B) 芙蓉洞和重庆主城区的位置;(C) 芙蓉洞示意图及监测点位置 (黑色三角形)。修改自参考文献[33]
Figure 1. (A) Location of the study area. Gray arrows: the Indian summer monsoon and the East Asian summer monsoon; white arrows: the Asian winter monsoon; red area: the location of Chongqing City); (B) Location of Furong Cave and the main urban area of Chongqing; (C) Sketch map of Furong Cave and the locations the locations of monitoring sites (black triangles). (modified from the previous study33)
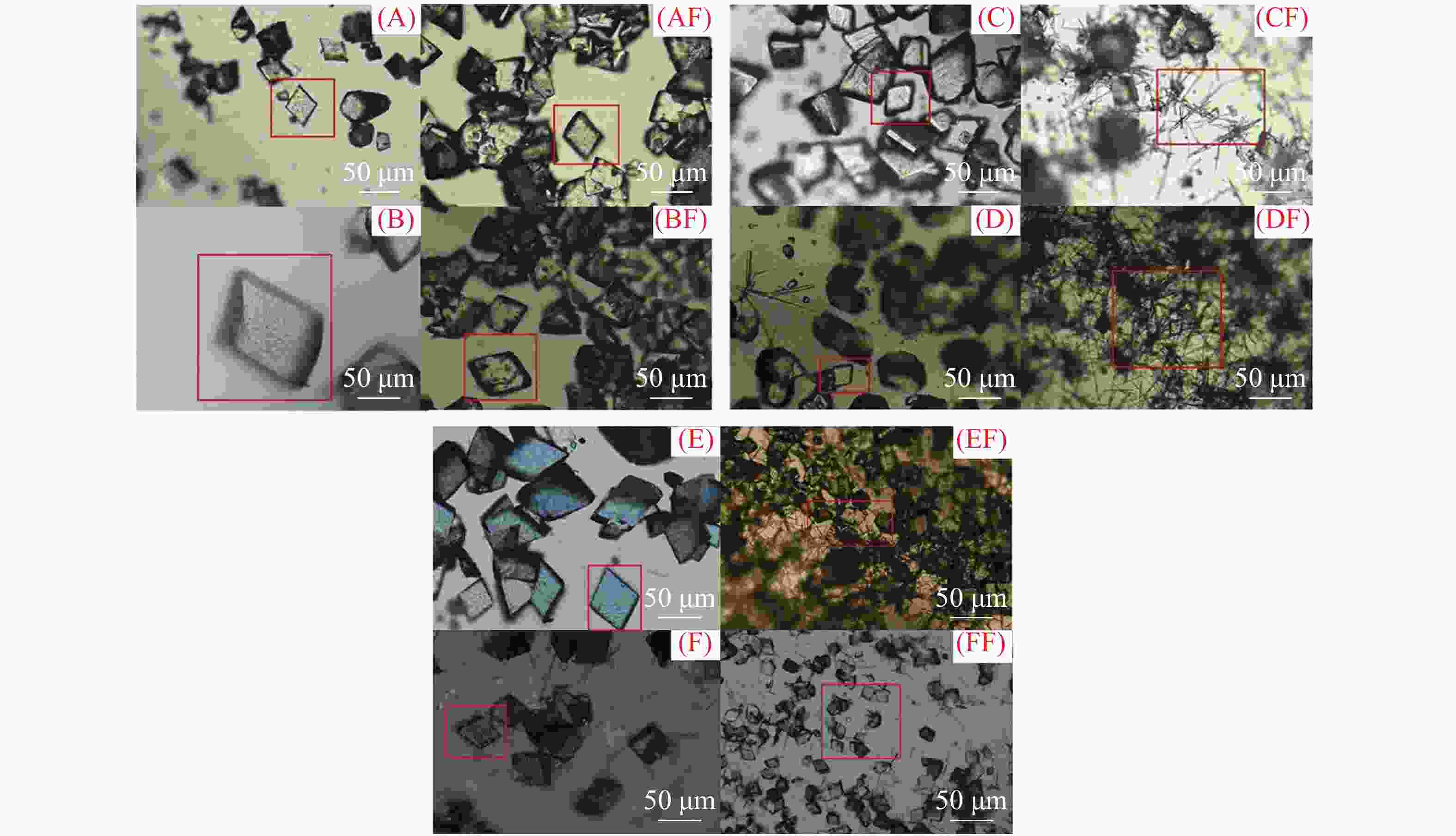
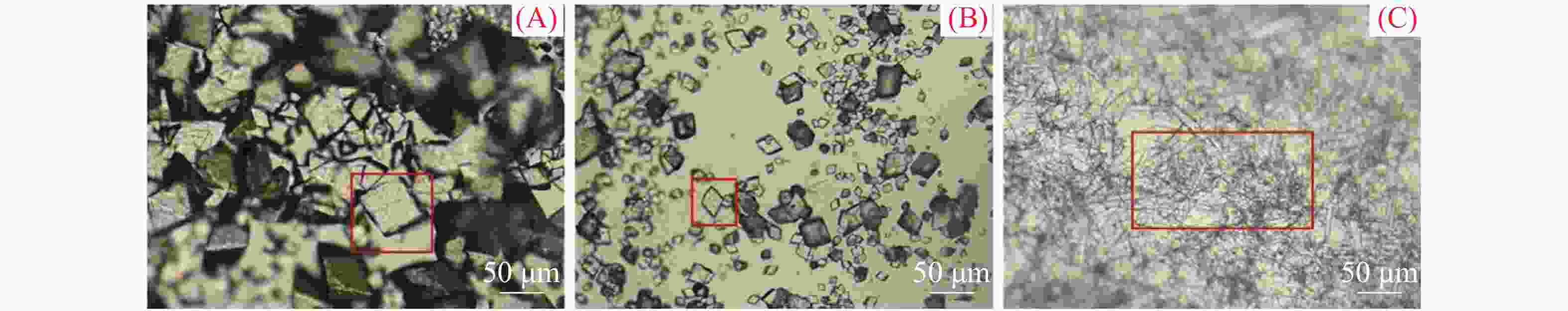
图 3 MP2、MP5、MP9新生碳酸钙沉积物矿物晶体形态
(A) MP2点玻璃片正面(2017年1−3月);(B) MP2点玻璃片正面(2017年4−6月);(C) MP5点玻璃片正面(2017年1−3月);(D) MP5点玻璃片正面(2017年7−9月);(E) MP9点玻璃片正面(2018年7−9月);(F) MP9点玻璃片正面(2018年10−12月);AF、BF、CF、DF、EF和FF均为对应沉积时段玻璃片反面新生碳酸钙沉积物的矿物形态
Figure 3. Mineral morphology of active speleothem at MP2, MP5 and MP9
(A) Front side of glass plate at MP2 (Jan.-Mar. 2017); (B) Front side of glass plate at MP2 (April-June 2017); (C) Front side of glass plate at MP5 (January-March 2017); (D) Front side of glass plate at MP5 (July-September 2017); (E) Front side of glass plate at MP9 (July-September 2018); (F) Front side of glass plate at MP9 (October-December 2018); AF, BF, CF, DF, EF and FF are mineral forms of AS on the back side of the glass plates during the corresponding deposition period
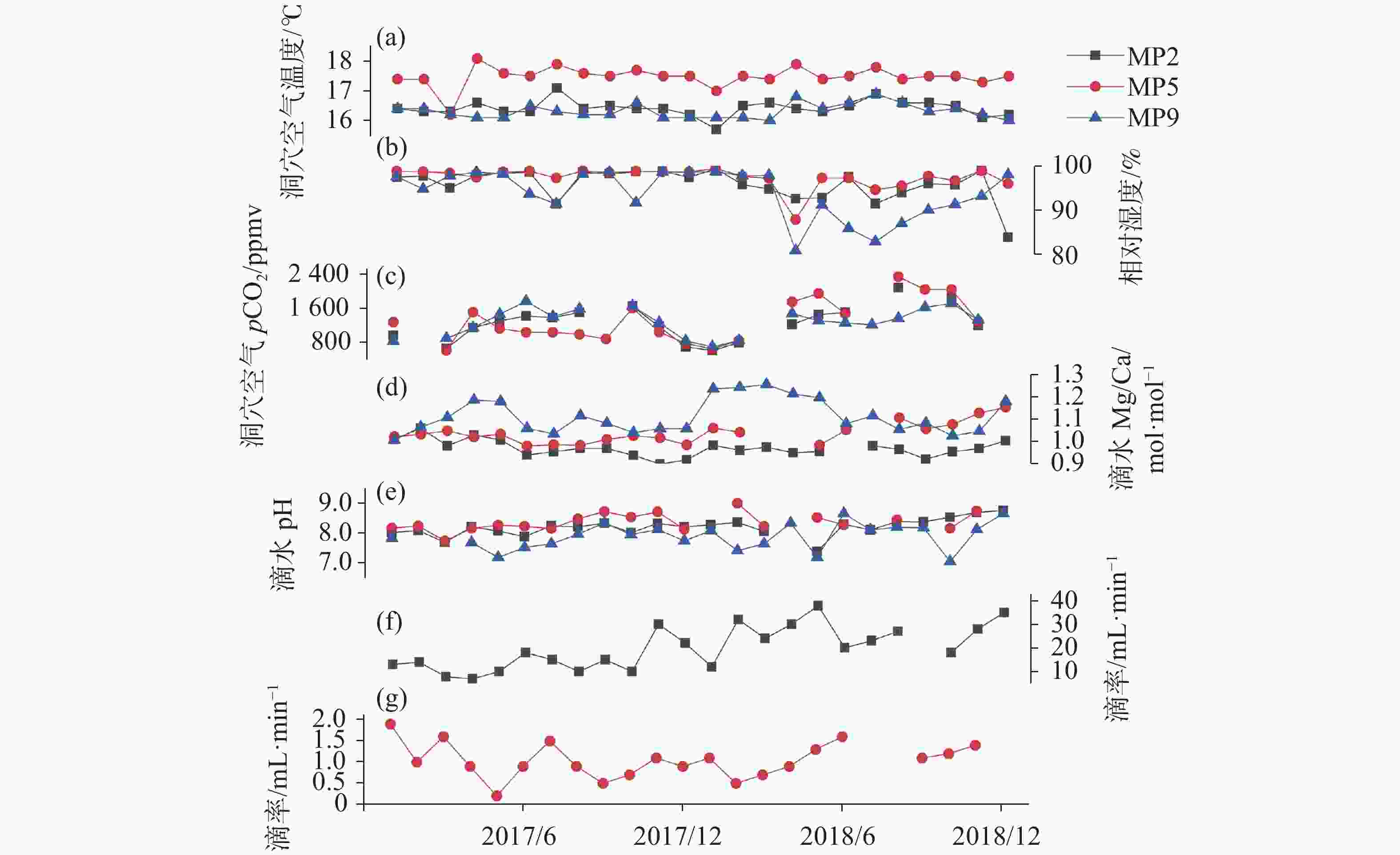
表 1 MP2、MP5和MP9滴水点的洞穴环境特征及滴水水化学特征
Table 1. Cave environmental characteristics and drip water chemical characteristics at MP2, MP5 and MP9
监测点 时间 温度
/℃湿度
/%pCO2
/10−6Mg2+
/mg·L−1Ca2+
/mg·L−1Mg/Ca
/mol·mol−1滴率
/mL·min−1pH MP2 201701 16.4 97.5 950 36.83 60.20 1.01 13 8.0 201702 16.3 97.7 — 37.29 58.04 1.06 14 8.1 201703 16.3 95.0 643 35.14 59.12 0.98 7.8 7.7 201704 16.6 97.9 1 132 36.86 59.14 1.03 6.9 8.2 201705 16.3 98.4 1 298 36.88 60.47 1.01 10 8.1 201706 16.3 98.6 1 413 33.89 59.57 0.94 18 7.9 201707 17.1 91.4 1 376 35.21 60.91 0.95 15 8.3 201708 16.4 98.6 1 498 34.42 58.65 0.97 10 8.2 201709 16.5 98.2 — 34.46 58.72 0.97 15 8.3 201710 16.4 98.7 1 646 33.96 59.80 0.94 10 8.0 201711 16.4 98.8 1 160 32.74 60.13 0.90 30 8.3 201712 16.2 97.4 686 33.17 59.70 0.92 22 8.2 201801 15.7 99.4 594 40.98 68.80 0.98 12 8.3 201802 16.5 95.8 783 41.36 71.06 0.96 32 8.4 201803 16.6 94.8 — 41.24 69.81 0.97 24 8.1 201804 16.4 92.6 1 216 40.61 70.58 0.95 30 — 201805 16.3 92.8 1 444 41.43 71.61 0.95 38 7.4 201806 16.5 97.5 1 499 — — — 20 8.3 MP2 201807 16.9 91.5 — 41.01 69.04 0.98 23 8.1 201808 16.6 94.0 2 085 40.76 69.73 0.96 27 8.4 201809 16.6 96.0 — 35.16 63.05 0.92 — 8.4 201810 16.5 95.7 1 839 35.49 61.40 0.95 18 8.5 201811 16.1 99.0 1 185 35.54 60.62 0.97 28 8.7 201812 16.2 83.8 — 35.53 58.42 1.00 35 8.8 MP5 201701 17.4 98.8 1 269 36.94 59.65 1.02 1.9 8.2 201702 17.4 98.7 — 37.01 59.17 1.03 1.0 8.2 201703 16.2 98.4 598 36.45 57.38 1.05 1.6 7.7 201704 18.1 97.4 1 505 37.79 61.10 1.02 0.9 8.2 201705 17.6 98.7 1 118 36.92 58.98 1.03 0.2 8.3 201706 17.5 98.8 1 029 34.71 58.44 0.98 0.9 8.2 201707 17.9 97.3 1 029 35.39 59.24 0.98 1.5 8.2 201708 17.6 98.9 977 34.76 58.35 0.98 0.9 8.5 201709 17.5 98.5 865 34.06 55.70 1.01 0.5 8.7 201710 17.7 98.8 1 600 34.64 55.75 1.02 0.7 8.5 201711 17.5 98.7 1 031 33.02 53.62 1.02 1.1 8.7 201712 17.5 98.7 765 34.15 57.23 0.98 0.9 8.1 201801 17.0 99.4 637 41.41 64.44 1.06 1.1 — 201802 17.5 97.7 831 40.62 64.37 1.04 0.5 9.0 201803 17.4 97.2 — — — — 0.7 8.2 201804 17.9 87.8 1 746 — — — 0.9 — 201805 17.4 97.3 1 947 44.51 74.65 0.98 1.3 8.5 201806 17.5 97.3 1 474 42.58 66.78 1.05 1.6 8.3 201807 17.8 94.6 — — — — — — 201808 17.4 95.5 2 344 41.42 61.76 1.11 — 8.5 201809 17.5 97.7 2 040 34.51 53.90 1.06 1.1 — 201810 17.5 96.7 2 040 35.12 53.81 1.08 1.2 8.2 201811 17.3 99.0 1 286 34.86 51.00 1.13 1.4 8.8 201812 17.5 96.0 — 34.11 48.75 1.15 — — MP9 201701 16.4 97.6 820 37.24 61.01 1.01 — 7.8 201702 16.4 94.8 — 37.85 58.66 1.06 — 7.8 201703 16.2 97.8 883 36.97 55.05 1.11 — — 201704 16.1 98.6 1 133 38.21 53.12 1.19 — 7.7 201705 16.1 98.2 1 454 36.18 50.58 1.18 — 7.2 201706 16.5 93.7 1 764 35.75 55.68 1.06 — 7.5 201707 16.3 91.4 1 395 35.87 57.24 1.03 — 7.6 201708 16.2 98.2 1 584 35.02 51.77 1.12 — 8.0 201709 16.2 98.6 — 34.76 53.00 1.08 — 8.4 201710 16.6 91.7 1 645 34.56 54.82 1.04 — 8.0 201711 16.1 98.6 1 253 34.63 54.01 1.06 — 8.1 201712 16.1 98.6 821 34.03 53.09 1.06 — 7.7 201801 16.1 98.7 685 42.40 56.52 1.24 — 8.1 MP9 201802 16.1 97.8 830 42.76 56.71 1.24 — 7.4 201803 16.0 97.9 — 42.50 55.78 1.26 — 7.6 201804 16.8 80.8 1 470 42.70 58.02 1.21 — 8.3 201805 16.4 91.2 1 302 44.71 61.66 1.20 — 7.2 201806 16.6 85.8 1 251 43.31 66.14 1.08 — 8.7 201807 16.9 82.8 1 206 41.89 61.93 1.12 — 8.1 201808 16.6 86.9 1 355 42.89 67.14 1.05 — 8.2 201809 16.3 90.0 1 619 35.33 53.82 1.08 — 8.2 201810 16.4 91.3 1 712 35.24 56.72 1.02 — 7.0 201811 16.2 93.2 1 317 35.36 55.79 1.04 — 8.1 201812 16.0 98.0 — 34.73 48.54 1.18 — 8.7 注:— 代表数据缺失。MP2 2018年6月水样丢失,MP5由于部分月份滴水量少,缺少滴水Mg2+、Ca2+、滴率和pH等数据,MP9滴水点2017-2018年未进行滴率监测,由于仪器故障部分洞穴空气pCO2数据缺失。 表 2 MP2、MP5和MP9滴水点下玻璃片正反面AS的矿物形态统计
Table 2. Mineral morphology statistics of AS on the front and back sides of glass plates at MP2, MP5 and MP9
监测点 正反面均为方解石 正面方解石,反面文石 正反面均有文石 MP2 所有 无 无 MP5 无 2017.01-03、2017.04-06、2017.10-12、
2018.01-03、2018.04-06、
2018.07-09、2018.10-122017.07-09 MP9 无 2017.07-09、2017.10-12、
2018.04-06、2018.07-092017.01-03
2017.04-06
2018.01-03
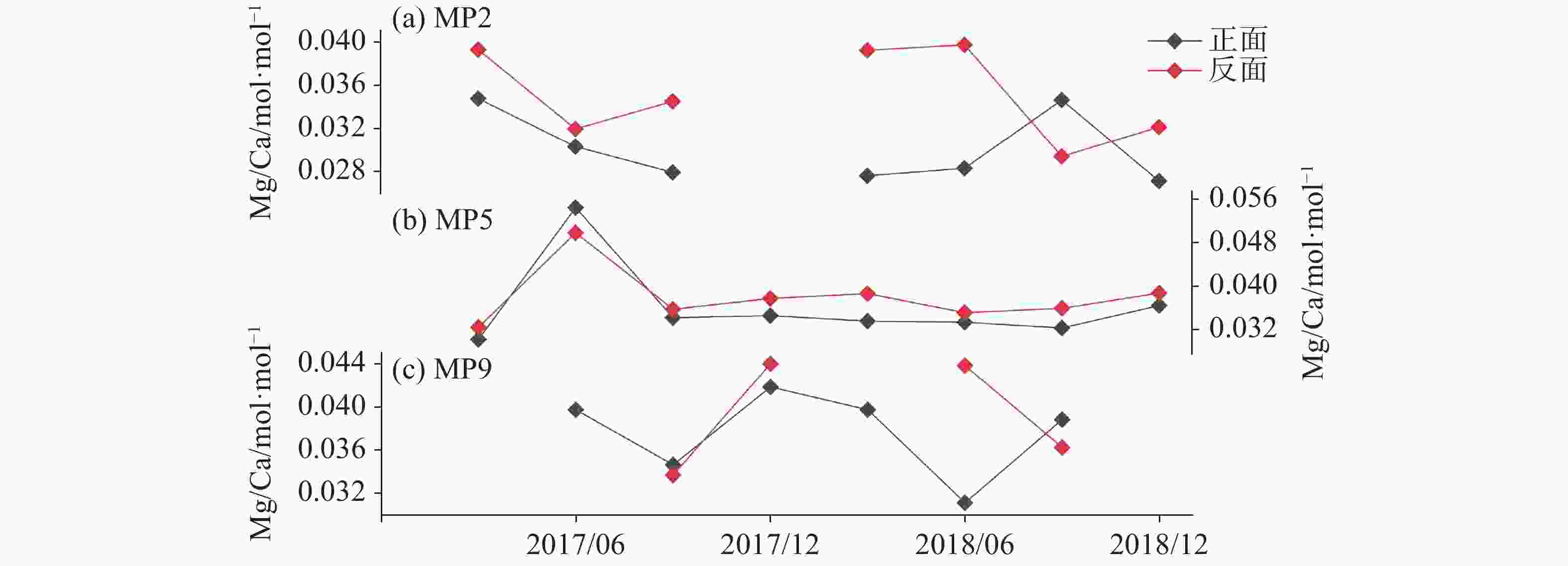
2018.10-12注:MP2缺少2017年10-12月的玻璃片。 表 3 玻璃片正反面新生碳酸钙沉积物的δ18O和δ13C及差值 (反面-正面)
Table 3. δ18O and δ13C in AS and the difference between the front and the back sides of glass plates
监测点 δ18O /‰, V-PDB δ13C /‰, V-PDB 沉积时间 正面 反面 差值 正面 反面 差值 MP2 −7.33 −6.95 0.38 −10.44 −10.13 0.31 2017.04−06 MP5 −7.48 −7.14 0.35 −10.28 −9.85 0.43 2018.10−12 MP9 −7.17 −7.18 −0.02 −9.95 −9.58 0.37 2017.07−09 MP9 −7.10 −6.75 0.36 −9.40 −9.12 0.28 2017.10−12 MP9 −7.30 −6.94 0.36 −9.73 −9.02 0.71 2018.04−06 MP9 −7.11 −6.79 0.32 −9.75 −8.78 0.97 2018.07−09 表 4 玻璃片正面新生碳酸钙沉积物的δ18O和δ13C值
Table 4. δ18O and δ13C values of AS on the front side of glass plates
沉积时间 MP2 MP5 MP9 δ18O
/‰,V-PDBδ13C
/‰,V-PDBδ18O
/‰,V-PDBδ13C
/‰,V-PDBδ18O
/‰,V-PDBδ13C
/‰,V-PDB2017.01-03 — — −6.95 −9.42 −6.51 −7.90 2017.04-06 −7.33 −10.44 −7.80 −10.96 −6.65 −9.23 2017.07-09 −7.05 −10.31 −7.54 −10.45 −7.17 −9.94 2017.10-12 — — −7.70 −10.96 −7.10 −9.40 2018.01-03 — — −7.53 −10.13 −6.69 −8.34 2018.04-06 — — −7.79 −10.57 −7.30 −9.73 2018.07-09 — — −7.63 −10.52 −7.11 −9.75 2018.10-12 — — −7.48 −10.28 — — -
[1] Wang Y J, Cheng H, Edwards R L, An Z S,Wu J Y, Shen C C, Dorale J A. A high-resolution absolute-dated late Pleistocene monsoon record from Hulu Cave, China[J]. Science, 2001, 294(5550):2345-2345. doi: 10.1126/science.1064618 [2] Bar-Matthews M, Ayalon A, Gilmour M, Matthews A,Hawkesworth C J. Sea–land oxygen isotopic relationships from planktonic foraminifera and speleothems in the Eastern Mediterranean region and their implication for paleorainfall during interglacial intervals[J]. Geochimica et Cosmochimica Acta, 2003, 67(17):3181-3199. doi: 10.1016/S0016-7037(02)01031-1 [3] Wang X, Auler A S, Edwards R L, Cheng H,Cristalli P S,Smart P L,Richards D A,Shen C C . Wet periods in northeastern Brazil over the past 210 kyr linked to distant climate anomalies[J]. Nature, 2004, 432(7018):740-743. doi: 10.1038/nature03067 [4] Denniston R F, Dupree M, Dorale J A, Asmerom Y,Polyak V J,Carpenter S J. Episodes of late Holocene aridity recorded by stalagmites from Devil’s Icebox Cave, central Missouri, USA[J]. Quaternary Research, 2007, 68(1):45-52. doi: 10.1016/j.yqres.2007.04.001 [5] Cheng H, Edwards R L, Broecker W S. Ice Age Terminations[J]. Science, 2009, 326(5950):248-252. doi: 10.1126/science.1177840 [6] 林玉石, 黄新耀, 张美良,覃家铭, 姜光辉, 朱晓燕, 杨琰, 向官生, 黄智勇. 中国南方发现大型文石笋[J]. 地学前缘, 2007(2):236-241. doi: 10.3321/j.issn:1005-2321.2007.02.020LIN Yushi, HUANG Xinyao, ZHANG Meiliang, QIN Jiaming, JIANG Guanghui, ZHU Xiaoyan, YANG Yan, XIANG Guansheng, HUANG Zhiyong. Large aragonite stalagmites found in south China[J]. Earth Science Frontiers, 2007(2):236-241. doi: 10.3321/j.issn:1005-2321.2007.02.020 [7] Murray J W. The deposition of calcite and aragonite in caves[J]. The Journal of Geology, 1954, 62(13):481-492. [8] Fairchild I J,Selmo E M, Borsato A,Mcdermott F,Frisia S. Aragonite-calcite relationships in speleothems (grotte de clamouse, france): environment, fabrics, and carbonate geochemistry[J]. Journal of Sedimentary Research, 2002, 72(5):687-699. doi: 10.1306/020702720687 [9] Davis K J. The role of Mg2+ as an impurity in calcite growth[J]. Science, 2000, 290(5494):1134-1137. doi: 10.1126/science.290.5494.1134 [10] Dove P M, Davis K J, De Yoreo J J, Wasylenki L E. Morphological consequences of differential Mg2+ incorporation at structurally distinct steps on calcite[J]. American Mineralogist, 2004, 89(5-6):714-720. doi: 10.2138/am-2004-5-605 [11] Riechelmann S, Schroeder Ritzrau A, Wassenburg J A, Schreuer J, Richter D K, Riechelmann D F C, Terente M, Constantin S, Mangini A, Immenhauser A. Physicochemical characteristics of drip waters: Influence on mineralogy and crystal morphology of recent cave carbonate precipitates[J]. Geochimica et Cosmochimica Acta, 2014, 145:13-29. doi: 10.1016/j.gca.2014.09.019 [12] Zhang H W, Cai Y J, Tan L C, An Z S. Stable isotope composition alteration produced by the aragonite-to-calcite transformation in speleothems and implications for paleoclimate reconstructions[J]. Sedimentary Geology, 2014, 309:1-14. doi: 10.1016/j.sedgeo.2014.05.007 [13] Burton E A, Walter L M. Relative precipitation rates of aragonite and Mg calcite from seawater: Temperature or carbonate ion control?[J]. Geology, 1987, 15(2):111-114. doi: 10.1130/0091-7613(1987)15<111:RPROAA>2.0.CO;2 [14] Railsback L B, Brook G A, Chen J, et al. Environmental controls on the petrology of a late Holocene speleothem from Botswana with annual layers of aragonite and calcite[J]. Journal of Sedimentary Research, 1994, 64(1a):147-155. [15] 张海伟, 蔡演军, 谭亮成. 石笋矿物类型、成因及其对气候和环境的指示[J]. 中国岩溶, 2010, 29(3):222-228. doi: 10.3969/j.issn.1001-4810.2010.03.002ZHANG Haiwei, CAI Yanjun, TAN Liangcheng. Phase composition and formation of stalagmite minerals: Indications of climate and environment[J][J]. Carsologica Sinica, 2010, 29(3):222-228. doi: 10.3969/j.issn.1001-4810.2010.03.002 [16] Fernández-Díaz L, Putnis A, Prieto M, Putnis C V. The role of magnesium in the crystallization of calcite and aragonite in a porous medium[J]. Journal of Sedimentary Research, 1996, 66:482-491. [17] Given R K, Wilkinson B H. Kinetic control of morphology, composition, and mineralogy of abiotic sedimentary carbonates[J]. Journal of Sedimentary Petrology, 1985, 55(1):109-119. [18] Fairchild I J, Tooth A F, Frisia S, Hawkesworth C J, Huang Y M, McDermott F,Borsato A. Controls on trace element (Sr–Mg) compositions of carbonate cave waters: implications for speleothem climatic records[J]. Chemical Geology, 2000, 166(3):255-269. [19] Harmon R S, Atkinson T C, Atkinson J L. The mineralogy of Castleguard Cave, Columbia Icefields, Alberta Canada[J]. Arctic and Alpine Research, 1983, 15(4):503-516. doi: 10.2307/1551236 [20] Fairchild I J, Treble P C. Trace elements in speleothems as recorders of environmental change[J]. Quaternary Science Reviews, 2009, 28(5-6):449-468. doi: 10.1016/j.quascirev.2008.11.007 [21] Caddeo G A, Waele J D, Frau F,Bruce Railsback L. Trace element and stable isotope data from a flowstone in a natural cave of the mining district of SW Sardinia (Italy): evidence for Zn2+-induced aragonite precipitation in comparatively wet climatic conditions[J]. International Journal of Speleology, 2011, 40(2):181-190. doi: 10.5038/1827-806X.40.2.10 [22] Wassenburg J A, Immenhauser A, Richter D K, Jochum K P, Fietzke J,Deininger M, Goos M, Scholz D,Sabaoui A. Climate and cave control on Pleistocene/Holocene calcite-to-aragonite transitions in speleothems from Morocco: Elemental and isotopic evidence[J]. Geochimica et Cosmochimica Acta, 2012, 92(1):23-47. [23] Wassenburg J A, Scholz D, Jochum K P, Cheng H,Oster J,Immenhauser A, Richter D K, Haeger T,Jamieson R A,Baldini J U L, Hoffmann D,Breitenbach S F M. Determination of aragonite trace element distribution coefficients from speleothem calcite-aragonite transitions[J]. Geochimica et Cosmochimica Acta, 2016, 190:347-367. doi: 10.1016/j.gca.2016.06.036 [24] Jens F, Jennifer A, Christoph S, Andrea S R, Birgit P, Christina G, Norbert F,Martin T. Carbon and oxygen isotope fractionation in the water-calcite-aragonite system[J]. Geochimica et Cosmochimica Acta, 2018:127-139. [25] Frisia S. Microstratigraphic logging of calcite fabrics in speleothems as tool for palaeoclimate studies[J]. International Journal of Speleology, 2014, 44(1):1-16. [26] Perrin C, Prestimonaco L, Servelle G, Tilhac R, Maury M, Cabrol P. Aragonite–Calcite Speleothems: Identifying Original and Diagenetic Features[J]. Journal of Sedimentary Research, 2014, 84(4):245-269. doi: 10.2110/jsr.2014.17 [27] Domínguez-Villar D, Krklec K, Pelicon P, Fairchild I J,Cheng H,Edwards L R. Geochemistry of speleothems affected by aragonite to calcite recrystallization – Potential inheritance from the precursor mineral[J]. Geochimica et Cosmochimica Acta, 2016, 200:310-329. [28] 杨琰, 袁道先, 程海,覃嘉铭,张美良,林玉石,朱晓燕. 文石-方解石石笋U/Th体系的封闭性判断及意义[J]. 地球化学, 2008, 37(2):97-106. doi: 10.3321/j.issn:0379-1726.2008.02.001YANG Yan, YUAN Daoxian, CHENG Hai, QIN Jiaming, ZHANG Meiliang, LIN Yushi, ZHU Xiaoyan. Discrimination of close U/Th system in aragonite-calcite stalagmites and their significance[J]. Geochimica, 2008, 37(2):97-106. doi: 10.3321/j.issn:0379-1726.2008.02.001 [29] 段武辉, 谭明, 程海, 张勇. 云南仙人洞文石类石笋年层结构电镜分析[J]. 第四纪研究, 2010, 30(5):1066-1067.DUAN Wuhui, TAN Ming, CHENG Hai, ZHANG Yong. Intra-annual Structure of Aragonitic Stalagmite Laminae from Yunnan Xianren Cave: SEM Analysis[J]. Quaternary Sciences, 2010, 30(5):1066-1067. [30] Duan W H, Cai B G, Tan M, Liu H, Zhang Y. The growth mechanism of the aragonitic stalagmite laminae from Yunnan Xianren Cave, SW China revealed by cave monitoring [J]. Boreas, 2012, 41(1): 113-123. [31] Ruan J Y,Hu C Y. Seasonal variations and environmental controls on stalagmite calcite crystal growth in Heshang Cave, central China[J]. Science Bulletin, 2010(34):83-89. [32] 朱学稳. 芙蓉洞的次生化学沉积物[J]. 中国岩溶, 1994, 13(4):357-368.ZHU Xuewen. Secondary speleothems in Furong Cave[J]. Carsologica Sinica, 1994, 13(4):357-368. [33] Li T Y, Shen C C, Li H C, Li J Y,Chiang H W,Song S R,Yuan D X,Lin C D J,Gao P, Zhou L, Wang J L,Ye M Y,Tang L L,Xie S Y. Oxygen and carbon isotopic systematics of aragonite speleothems and water in Furong Cave, Chongqing, China[J]. Geochimica et Cosmochimica Acta, 2011, 75(15):4140-4156. doi: 10.1016/j.gca.2011.04.003 [34] LI T Y,LI H C,XIANG X J ,KUO Tz S,LI J Y,ZHOU F L,CHEN H L,PENG L L. Transportation characteristics of δ13C in the plants-soil-bedrock-cave system in Chongqing karst area[J]. Science China, 2012, 55(4):685-694. doi: 10.1007/s11430-011-4294-y [35] Zhang J, Li T Y. Seasonal and interannual variations of hydrochemical characteristics and stable isotopic compositions of drip waters in Furong Cave, southwest China based on 12years' monitoring[J]. Journal of Hydrology, 2019, 572:40-50. doi: 10.1016/j.jhydrol.2019.02.052 [36] 黄春霞, 李廷勇, 韩立银, 李俊云, 袁娜, 王海波, 张涛涛, 赵鑫. 重庆芙蓉洞滴水现代次生化学沉积物沉积速率与元素特征[J]. 中国岩溶, 2015, 34(3):238-246.HUANG Chunxia, LI Tingyong, HAN Liyin, LI Junyun, YUAN Na, WANG Haibo, ZHANG Taotao, ZHAO Xin. Deposition rates and element features of active sediments under drip water in Furong cave of Chongqing[J]. Carsologica Sinica, 2015, 34(3):238-246. [37] 周福莉, 李廷勇, 陈虹利, 彭玲莉,李俊云,代彪. 重庆芙蓉洞洞穴水水文地球化学指标的时空变化[J]. 水土保持学报, 2012, 26(3):253-259.ZHOU Fuli, LI Tingyong, CHEN Hongli, PENG Lingli, LI Junyun, DAI Biao. Spacial and temporal variation of hydrogeochemical indices of the cave water in Furong cave, Chongqing[J]. Jourual of Soil and Water Conservation, 2012, 26(3):253-259. [38] Li J Y, Li T Y. Seasonal and annual changes in soil/cave air pCO2 and the δ13C DIC of cave drip water in response to changes in temperature and rainfall[J]. Applied Geochemistry, 2018, 93:94-101. doi: 10.1016/j.apgeochem.2018.04.002 [39] Berner R A. The role of magnesium in the crystal growth of calcite and aragonite from sea water[J]. Geochimica et Cosmochimica Acta, 1975, 39:489-504. doi: 10.1016/0016-7037(75)90102-7 [40] Thrailkill J. Carbonate deposition in Carlsbad caverns[J]. The Journal of Geology, 1971, 79(6):683-695. doi: 10.1086/627698 [41] Cabrol P, Coudray J. Climatic fluctuations influence the genesis and diagenesis of carbonate speleothems in southwestern France[J]. National Speleological Society Bulletin, 1982, 44(4):112-117. [42] Li J Y, Li T Y, Shen C C, et al. Variations and significance of Mg/Sr and 87Sr/86Sr in a karst cave system in southwest China[J]. Journal of Hydrology, 2021, 596:126140. doi: 10.1016/j.jhydrol.2021.126140 [43] Spötl C, Unterwurzacher M, Mangini A, Mangini A. Carbonate Speleothems in the Dry, Inneralpine Vinschgau Valley, Northernmost Italy: Witnesses of Changes in Climate and Hydrology Since the Last Glacial Maximum[J]. Journal of Sedimentary Research, 2002, 72(6):793-808. doi: 10.1306/041102720793 [44] Mcmillan E A, Fairchild I J, Frisia S, et al. Annual trace element cycles in calcite–aragonite speleothems: evidence of drought in the western Mediterranean 1200–1100yrBP [J]. Journal of Quaternary Science, 2005, 20 (5). [45] Csoma A E, Goldstein R H, Pomar L. Pleistocene speleothems of Mallorca: implications for palaeoclimate and carbonate diagenesis in mixing zones[J]. Sedimentology, 2006, 53(1):213-236. doi: 10.1111/j.1365-3091.2005.00759.x [46] Musgrove M L and Banner J L. Controls on the spatial and temporal variability of vadose dripwater geochemistry: Edwards aquifer, central Texas[J]. Geochimica et Cosmochimica Acta, 2004, 68(5):1007-1020. doi: 10.1016/j.gca.2003.08.014 [47] Rossi C, Lozano R P. Hydrochemical controls on aragonite versus calcite precipitation in cave dripwaters[J]. Geochimica et Cosmochimica Acta, 2016, 192:70-96. doi: 10.1016/j.gca.2016.07.021 [48] Tarutani T, Clayton R N, Mayeda T K. The effect of polymorphism and magnesium substitution on oxygen isotope fractionation between calcium carbonate and water[J]. Geochimica et Cosmochimica Acta, 1969, 33(8):987-996. doi: 10.1016/0016-7037(69)90108-2 [49] Mucci A, O’Neil J R, Kim S T,Hillaire-Marcel C. Oxygen isotope fractionation between synthetic aragonite and water: Influence of temperature and Mg2+ concentration[J]. Geochimica et Cosmochimica Acta, 2007, 71(19):4704-4715. doi: 10.1016/j.gca.2007.04.019 [50] Grossman E L, Ku T L. Aragonite-isotopic paleotemperature scale based on the benthic foraminifera Hoeglundina elegans [J], 1981, 13: 464. [51] Rubinson M, Clayton R N. Carbon-13 fractionation between aragonite and calcite[J]. Geochimica et Cosmochimica Acta, 1969, 33(8):997-1002. doi: 10.1016/0016-7037(69)90109-4 [52] Mickler P J, Stern L A, Banner J L. Large kinetic isotope effects in modern speleothems[J]. Geological Society of America Bulletin, 2006, 118(1-2):65-81. doi: 10.1130/B25698.1 [53] Deininger M, Fohlmeister J, Scholz D, Mangini A. Isotope disequilibrium effects: The influence of evaporation and ventilation effects on the carbon and oxygen isotope composition of speleothems: A model approach[J]. Geochimica et Cosmochimica Acta, 2012, 96(1):57-79. [54] Feng W M, Casteel R C, Banner J L,Ayla Heinze-Fry. Oxygen isotope variations in rainfall, drip-water and speleothem calcite from a well-ventilated cave in Texas, USA: Assessing a new speleothem temperature proxy[J]. Geochimica et Cosmochimica Acta, 2014, 127:233-250. doi: 10.1016/j.gca.2013.11.039 [55] Chen C J,Huang R,Yuan D X,Zhang J,Cheng H,Ning Y F,Yu T L, Shen C C ,Edwards R L,Long X Y,Wang T,Xiao S Y,Wu Y,Liu Z Q,Li T Y,Li J Y. Karst hydrological changes during the Late-Holocene in Southwestern China[J]. Quaternary Science Reviews, 2021, 258:106865. doi: 10.1016/j.quascirev.2021.106865 -




 下载:
下载: