Carbon sink of microalgae in karst lakes under the influence of the extracellular of carbonic anhydrase
-
摘要: 以岩溶湖泊——红枫湖的微藻为研究对象,通过添加两种标记稳定碳同位素组成的无机碳进行室内模拟岩溶环境条件;并通过添加不同浓度的乙酰唑胺(AZ),来模拟岩溶湖泊中碳酸酐酶胞外酶活性差异的各类微藻。重点监测微藻蛋白质含量及其稳定碳同位素组成变化等指标,计算其对不同来源无机碳的吸收利用份额,并结合微藻的生物量生长指标,最终计算出碳酸酐酶胞外酶活性差异的各种微藻的碳汇能力。结果显示:在岩溶湖泊的自然水体中,碳酸酐酶胞外酶活性强的微藻碳汇能力是缺乏碳酸酐酶胞外酶的微藻碳汇能力的5倍。碳酸酐酶胞外酶对微藻光合碳汇能力的影响显著。Abstract:
Carbonic anhydrase is a metal enzyme which contains Zn. It has the characteristics of catalyzing the mutual conversion between CO2 and HCO3− with high efficiency and specificity. It plays an important role in promoting the global carbon cycle, such as carbonate dissolution, photosynthesis of plants and atmospheric CO2 hydration reaction. Karst carbon sink refers to the biological carbon sequestration process which is the aquatic organisms represented by microalgae absorb and utilize inorganic carbon represented by HCO3− from carbonate karst erosion. In karst lake, under the catalytic action of carbonic anhydrase, it can greatly accelerate the dissolution process of carbonate rock, significantly affect the pH and the concentration of HCO3− of karst lake water,and promote the growth of microalgae. Correspondingly, the karst carbon sink capacity of microalgae increased with the increasing of biomass. And on this basis, the life activities of microalgae can promote the dissolution process of the karstification. Finally, it forms an aquatic photosynthetic carbon cycling system between carbonate rocks and atmosphere with the participation of microalgae. Isotopes are different atoms of the same element with the same number of protons but different numbers of neutrons. Isotopes in nature can be divided into radioisotopes and stable isotopes according to their stability. Stable isotope analysis is accurate, pollution-free and non-destructive, which can be used to study the interaction between organisms and the environment. It has been widely used in the field of plant ecology.Stable isotopes were used in this study. Microalgae is the primary producer of aquatic ecosystem, which refers to a class of microscopic plants living in water and living in a planktonic lifestyle. The character of microalgae in karst lakes have typical seasonal fluctuation and spatial heterogeneity. The activity of carbonic anhydrase was different significantly among various microalgae. In spring and summer, green algae dominated with strong carbonic anhydrase activity and fast growth; while in autumn and winter, the dominated algae is the diatoms which with weak carbonic anhydrase activity and slow growth. In conclusion, the activity of carbonic anhydrase determines the ability of microalgae to obtain inorganic carbon, which brings about great differences in the growth of various microalgae,and then affects a huge impact in the capacity of photosynthetic carbon sink in microalgae. Acetazolamide (AZ) belongs to the sulfonamide group which is a specific inhibitor about the extracellular of carbonic anhydrase. Its substrate is CAex, and it has a good inhibitory effect on CAex. In this study, the microalgae in Hongfeng lake, a karst plateau lake,was taken as the research object, we simulated karst condition in laboratory by adding different inorganic carbon labeled. In addition, different concentrations of AZ were added to simulate the difference of carbonic anhydrase extracellular enzyme activity of different microalgae of karst lakes. By monitoring the protein content and stable carbon isotope composition of microalgae, the proportion of absorption and utilization of inorganic carbon from different sources was calculated. The carbon sink of microalgae with different extracellular enzyme activities were calculated based on the biomass growth index of microalgae and the above proportion. The results showed that the carbon sink capacity of microalgae with high activity of carbonic anhydrase was 5 times higher than that of microalgae without carbonic anhydrase in natural water of karst lake. The extracellular carbonic anhydrase had significant effects on the photosynthetic carbon sink capacity of microalgae. Although the content of native bicarbonate is high in karst lake water, inorganic carbon utilized by microalgae mainly comes from atmospheric carbon dioxide (photosynthetic carbon sink), and only a small amount of native bicarbonate (karst carbon sink) is utilized in karst lake water. The main contribution of carbonic anhydrase extracellular enzyme is accelerating the absorption, utilization and conversion efficiency of atmospheric carbon dioxide by microalgae, and finally promoted the growth of microalgae, carbon sequestration and increase sink. It is of great significance for scientific selection of human using microalgae with strong extracellular enzyme activity of carbonic anhydrase to increase carbon sink and serve the national strategy of "carbon neutrality". -
Key words:
- microalgae /
- karst carbon sink /
- carbonic anhydrase /
- karst lake
-
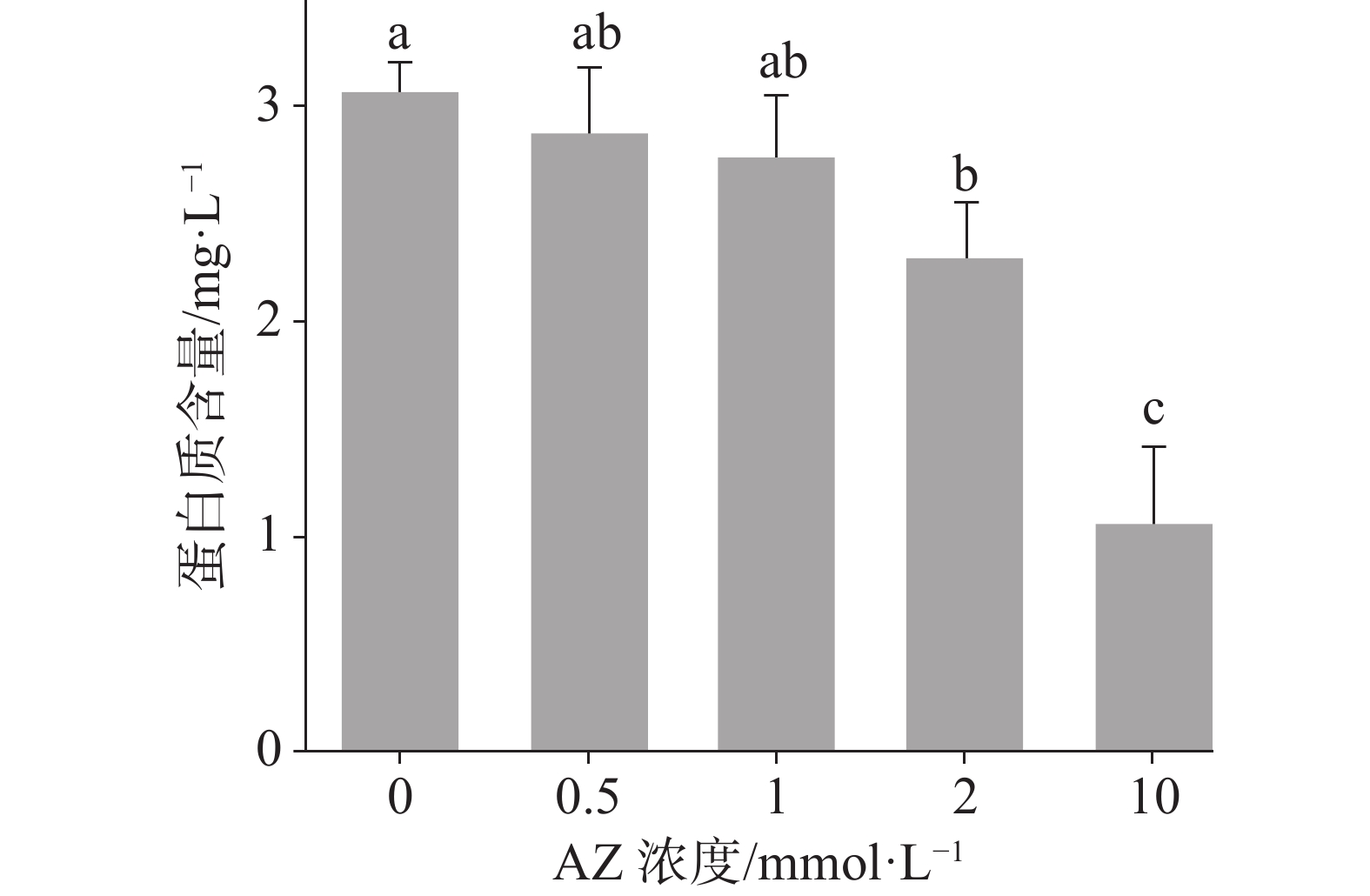
表 1 AZ浓度梯度处理下的微藻碳同位素组成(‰, PDB)
Table 1. δ13C value of the microalgae under different concentrations of AZ (‰, PDB)
[AZ]a (mmol·L−1) δT1 δT2 0 −27.6±0.1 −28.3±0.1 0.5 −29.1±0.2 −30.2±0.2 1.0 −29.3±0.3 −30.6±0.2 2.0 −29.8±0.3 −31.3±0.4 10.00 −32.3±0.5 −33.7±0.5 [AZ]a-培养液中添加的AZ浓度; δT1-添加δ13C 为-17.4‰的 NaHCO3培养液; δT2-添加δ13C 为-28.4‰的 NaHCO3培养液; 表中数据为平均值±标准差(n=3)。 表 2 AZ浓度梯度处理下的微藻对不同碳源的利用及碳汇估算
Table 2. Utilization of different carbon source and estimation of carbon sinks by microalgae under different concentrations of AZ
[AZ]a /mmol·L−1 M fB CSk CSp P/% 0 6.13 0.06 0.33 4.80 100.00 0.5 5.75 0.10 0.47 4.28 88.99 1.0 5.51 0.12 0.53 3.98 83.15 2.0 4.58 0.14 0.49 3.09 64.41 10.00 2.10 0.13 0.14 0.96 19.88 [AZ]a-培养液中添加的AZ浓度; M-微藻生物量的增殖倍数; fB-微藻吸收利用培养液中添加的碳源的份额 ;CSk-岩溶碳汇能力; CSp-光合碳汇能力; P-相比于未添加AZ的湖泊自然状态下的微藻碳汇能力的比例。 -
[1] Khalifah R G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C[J]. Journal of Biological Chemistry, 1971, 246(8):2561-2573. doi: 10.1016/S0021-9258(18)62326-9 [2] 吴沿友, 李海涛, 谢腾详. 微藻碳酸酐酶生物地球化学作用[M]. 北京: 科学出版社, 2015, 1-251.WU Yanyou, LI Haitao, XIE Tengxiang. Biogeochemical Action of Microalgal Carbonic Anhydrase [M]. Beijing: Science Press, 2015: 1-251. [3] LIU Zaihua, Dreybrodt Wolfgang. Significance of the carbon sink produced by H2O-carbonate-CO2-aquatic phototroph interaction on land[J]. Science Bulletin, 2015, 2(60):182-191. [4] 张陶, 李建鸿, 蒲俊兵, 李瑞, 吴飞红, 李丽.小球藻对岩溶水体Ca2+、HCO3− 利用效率实验研究[J]. 中国岩溶, 2018, 37(1): 81-90.ZHANG Tao, LI Jianhong, PU Junbing LI Rui, WU feihong, LI Li. Experimental study on the utilization efficiency of Chlorella to Ca2+ and HCO3− in karst water[J]. Carsologica Sinica, 2018, 37(1): 81-90. [5] 黄炳惠, 李强, 房君佳, 曹建华, 靳振江, 彭文杰, 卢晓漩, 梁月明. CO2浓度梯度对两种岩溶微藻碳酸酐酶活性的影响[J]. 中国岩溶, 2018, 37(1):91-100.HUANG Binghui, LI Qiang, FANG Junjia, CAO Jianhua, JIN Zhenjiang, PENG Wenjie, LU Xiaoxuan, LIANG Yueming. Effects of CO2 concentration gradient on carbonic anhydrase of two karst microalgae[J]. Carsologica Sinica, 2018, 37(1):91-100. [6] 余龙江, 吴云, 李为, 曾宪东, 付春华. 微生物碳酸酐酶对石灰岩的溶蚀驱动作用研究[J]. 中国岩溶, 2004, 23(3): 225-228.YU Longjiang, WU Yu, LI Wei, ZHENG Xiandong, FU Chunhua. Study on the driving effects on limestone corrosion by microbial carbonic anhydrase[J]. Carsologica Sinica, 2004, 23(3): 225-228. [7] 张小菊, 杨翠珍, 杨娟. 微生物碳酸酐酶在岩溶发育中的研究现状及展望[J]. 化学与生物工程, 2011, 28(2): 9-11.ZHANG Xiaoju, YANG Cuizhen, YANG Juan. The status and prospect of microbial carbonic anhydrase research in karst development[J]. Chemistry and bioengineering, 2011, 28(2): 9-11. [8] PU Junbing, LI Jianhong, Khadka M B, Martin J B, ZHANG Tao, YU Shi, YUAN Daoxiang. In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream[J]. Science of the Total Environment, 2017, 579:1343-1355. doi: 10.1016/j.scitotenv.2016.11.132 [9] Bell T AS, Sen-Kilic Emel, Felföldi Tamás, Gabor V, Fields M W, Peyton B M. Microbial community changes during a toxic cyanobacterial bloom in an alkaline Hungarian lake[J]. Antonie van Leeuwenhoek, 2018, 111(12):2425-2440. doi: 10.1007/s10482-018-1132-7 [10] Deepa, P K, Panneerselvam, A, Thajuddin, N. Seasonal variation of planktonic microalgal and cyanobacterial diversity in the temple pond of Tepakulam, Tiruchirappalli, Tamil Nadu[J]. Zenith International Journal of Multidisciplinary Research, 2019, 9(3):23-28. [11] 黄国佳, 李秋华, 陈椽, 商立海, 张垒, 欧滕, 高廷进, 高钥, 邓龙. 贵州高原红枫湖水库浮游植物功能分组及其时空分布特征[J]. 生态学报, 2015, 35(17):1-12.HUANG Guojia, LI Qiuhua, CHEN Chan, SHANG Lihai, ZHANG Lei, OU Teng, GAO Tingjin, GAO Yue, DENG Long. Phytoplankton functional groups and their spatial and temporal distribution characteristics in Hongfeng reservoir, Guizhou province[J]. Acta Ecologica Sinica, 2015, 35(17):1-12. [12] Fry B, E B Sherr. δ13C measurements as indicators of carbon flow on marine and freshwater ecosystems[J]. Contributions in Marine Science, 1984, 27:13-47. [13] Darren L Bade, Michael L Pace, Jonathan J Cole, Stephen R Carpenter. Can algal photosynthetic inorganic carbon isotope fractionation be predicted in lakes using existing models?[J]. Aquatic Sciences, 2006, 68(2):142-153. doi: 10.1007/s00027-006-0818-5 [14] CHEN Zhen, CHENG Huimin, CHEN Xiongwen. Effect of Cl− on photosynthetic bicarbonate uptake in two cyanobacteria Microcystis aeruginosa and Synechocystis PCC6803[J]. Chinese Science Bulletin, 2009, 54(7):1197-1203. [15] Mook W G, Bommerson J C, Staverman W H. Carbon Isotope Fractionation Between Dissolved Bicarbonate and Gaseous Carbon Dioxide[J]. Earth and Planetary Science Letters, 1974, 22(2):169-176. doi: 10.1016/0012-821X(74)90078-8 [16] Marlier J F, O"Leary M H. Carbon kinetic isotope effects on the hydration of carbon dioxide and the dehydration of bicarbonate ion[J]. Journal of the American Chemical Society, 1984, 106(18):5054-5057. doi: 10.1021/ja00330a003 [17] WU Yanyou, XU Ying, LI Haitao, XING Deke. Effect of acetazolamide on stable carbon isotope fractionation in Chlamydomonas reinhardtii and Chlorella vulgaris[J]. Chinese Science Bulletin, 2012, 57(7):786-789. doi: 10.1007/s11434-011-4861-9 [18] 李海涛, 吴沿友, 谢腾祥. 微藻利用不同无机碳途径的定量方法[J]. 地球与环境, 2014, 42(1):116-121. doi: 10.14050/j.cnki.1672-9250.2014.01.002LI Haitao, WU Yanyou, XIE Tengxiang. The method of quantifying inorganic carbon pathways in microalga[J]. Earth and Environment, 2014, 42(1):116-121. doi: 10.14050/j.cnki.1672-9250.2014.01.002 [19] 曲春香, 沈颂东, 王雪峰, 崔永华, 宋卫平. 用考马斯亮蓝测定植物粗提液中可溶性蛋白质含量方法的研究[J]. 苏州大学学报(自然科学版), 2006, 22(2):82-85.QU Chunxiang, SHEN Songdong, WANG Xuefeng, CUI yonghua, SONG Weiping. Method research of measuring soluble protein contents of plant rough extraction using Coomassie Brilliant Blue[J]. Journal of Suzhou university (Natural science edition), 2006, 22(2):82-85. [20] 李海涛, 吴沿友, 赵丽华, 张开艳, 杭红涛. 双同位素示踪定量微藻对碳源利用份额的方法研究[J]. 中国岩溶, 2016, 35(6):614-618.LI Haitao, WU Yanyou, ZHAO Lihua, ZHANG Kaiyan, HANG Hongtao. Application of bidirectional labeling method to quantifying carbon utilization in microalgae[J]. Carsologica Sinica, 2016, 35(6):614-618. [21] 徐涛, 宋立荣. 三株铜绿微囊藻对外源无机碳利用的研究[J]. 水生生物学报, 2007, 31(2):245-250. doi: 10.3321/j.issn:1000-3207.2007.02.016XU Tao, SONG Lirong. Studies on the utility of inorganic carbon in three strains of microcystis aeruginosa[J]. Acta Hydrobiologica Sinica, 2007, 31(2):245-250. doi: 10.3321/j.issn:1000-3207.2007.02.016 [22] 吴雁雯, 张金池. 微生物碳酸酐酶在岩溶系统碳循环中的作用与应用研究进展[J]. 生物学杂志, 2015, 32(3): 78-83.WU Yanwen, ZHANG Jinchi, Microbial carbonic anhydrase action and application on carbon cycling in karst dynamic system: a review[J]. Journal of Biology, 2015, 32(3): 78-83. [23] 蒋忠诚, 袁道先, 曹建华, 覃小群, 何师意, 章程. 中国岩溶碳汇潜力研究[J]. 地球学报, 2012, 33(2):129-134.JIANG Zhongcheng, YUAN Daoxian, CAO Jianhua. QIN Xiaoqun, HE Shiyi, ZHANG Cheng, A Study of carbon sink capacity of karst processes in China[J]. Acta Geoscientica Sinica, 2012, 33(2):129-134. -




 下载:
下载: